Dietary shochu kasu alleviates fatty liver induced by orotic acid
Abstract
The effects of dietary shochu kasu (5%) on orotic acid-induced fatty liver were studied in rats. Liver triglyceride content of orotic acid-fed rats increased by 5Âfold in comparison to basal group. Shochu kasu reduced triglyceride content by two-third. The activities of fatty acid synthase and phosphatidate phosphohydrolase significantly decreased, that was approximately by 35 and 20%, respectively. The serum lipid levels however were nearly unchanged. The alleviation of fatty liver in rats with shochu kasu, therefore, was associated with the inhibition of fatty acid synthase and phosphatidate phosphohydrolase activities.
Introduction
Liver is a main organ of lipid metabolism (Harrison and Diehl, 2002). The excesses accumulation of lipids in the liver with a cut-off level exceeds 5-10% of the liver weight is known as simple fatty liver or hepatic steatosis (Sherlock and Dooley, 1997). The certain chemical compounds, nutritional, and endocrine disorders can cause hepatic steatosis. Orotic acid can induce hepatic steatosis (Cha et al., 1998; Buang et al., 2004; Buang et al., 2005) which is similar to those seen in patients with alcoholic liver disease and range from mild hepatic steatosis to steatohepatitis, liver fibrosis, and cirrhosis (Dixon et al., 2001) and, rarely, to hepatocellular carcinoma (Cotrim et al., 2000).
Shochu is produced from material rich in carbohydrate such as rice, buckwheat, barley, potato, or cane. The shochu distillery waste, a by product of shochu production, is known as shochu kasu (Yokoyama et al., 2002). Ten liters of shochu produced from the raw materials releases 15 kg waste that have high concentration of organic substances. The wastewaters released from shochu manufacture have high chemical oxygen
demand values, in which it ranges between 60,000 and 90,000 mg/L (Tsushin, 2005). Furthermore, shochu kasu also has prebiotic effects because it contains useful bioactive substances such as growth-stimulating factor (Yokoyama et al., 2001). Shochu kasu contains a significant amount of linoleic acid, a polyunsaturated fatty acids (PUFA). PUFA inhibited the activity of fatty acid synthase (Moon et al., 2002). Fatty acid synthase is the key enzyme of fatty acid biosynthesis. Shochu kasu, therefore, might have beneficial function to ameliorate triglyceride accumulation in the liver. The present study was conducted to know the effects of dietary shochu kasu in fatty liver induced by orotic acid using Sprague-Dawley rats as animal model.
Materials and Methods
Animals, diets, and experimental design
All aspects of the experiment were conducted according to guidelines provided by the ethical committee of experimental animal care at Saga University. Male Sprague-Dawley rats (5 weeks) were housed individually in an air-conditioned room (24°C) with a 12 hours light/dark cycle. After one week adaptation period, rats were assigned to one of three groups (five rats each). Basal diets were prepared according to recommendations of the American Institute of Nutrition and contained (in weight %) 20 of casein, 7 of soybean oil (palmitic acid 10%, oleic acid 25%, and linoleic acid 55%), 1 of vitamin mixture (AIN-93), 3.5 of mineral mixture (AIN-93), 0.25 of choline bitartrate, 0.3 of L-cystine, 0.0014 of ter-butylhydroquinone, 5 of cellulose, 10 of sucrose, 13.2 of a-cornstarch, and β-cornstarch, to make 100. The orotic acid and shochu kasu diets were prepared by supplementation of 1% orotic acid and 5% shochu kasu (Iki Shochu Kyogyo Kumiai), respectively, to the basal diet at the expense of β-cornstarch. The animals received the diets for 10 days. On day 11, rats were killed by decapitation. Livers were excised immediately, and serum was separated from blood.
Analysis of lipids
Liver lipids were extracted (Folch et al., 1957) and concentrations of triglyceride and phospholipid were measured by the methods described elsewhere (Fletcher, 1968; Bartlett, 1959). Serum triglyceride, phospholipid, and total cholesterol were measured using enzyme assay kits (Wako Pure Chemicals) instructions.
Preparation of liver subcellular fractions
The mitochondrial, cytosol, and microsome of liver sub cellular fractions were prepared as described previously (Ikeda et al., 1998). Protein concentration was determined by the method of Lowry et al. (1951).
Assays of hepatic enzyme activity
The enzyme activities of fatty acid synthase (EC2.3.1.85) and phosphatidic acid (phosphatidate) phosphohydrolase (EC3.1.3.4) were determined as previously described (Ikeda et al., 1998; Walton et al., 1985).
Statistical analyses
Data were analyzed by one-way analysis of variance, and all differences were inspected by Duncan's new multiple-range test using SPSS statistical software.
Results
The daily intake of food was paired-fed for each animal in order to get the same quantity of macronutrient, vitamin, and mineral ingested. The macronutrient as sources of caloric food of each group was prepared in excess amount. It is therefore, the addition of food supplements, both orotic acid and shochu kasu, to the basal diet provides the same quantity of the energy needed by each animal's homeostasis metabolisms of each group during the time course. Thus, the effect of shochu kasu intake in orotic acid-induced fatty liver by the given concentration can be elucidated from this
experimental design. Although food intakes were nearly similar among the groups, the final body weights of orotic acid-treated rats decreased slightly (Table I). It was however the weight of liver in the group increased significantly. The slightly decreased final body weight of shochu kasu-containing group than that of orotic acid group might be contributed by the reduction of white adipose tissues weight, mainly omental and perirenal {(omental; orotic acid + shochu kasu, 1.23 ± 0.02; orotic acid, 1.42 ± 0.07) (perirenal; orotic acid + shochu kasu, 0.66 ± 0.07; orotic acid, 0.96 ± 0.12)}. Furthermore, dietary orotic acid promoted liver weight significantly than that of the basal group. Although failed to reach significant level, the addition of shochu kasu in orotic acid-treated rats attenuated the weight of liver by 3%.
Table I: Effects of dietary shochu kasu on growth parameters of orotic acid-treated rats
| Group | Basal | Orotic acid | Orotic acid + Shochu kasu |
|---|---|---|---|
| Initial body weight (g) | 133 ± 3 | 133 ± 3 | 134 ± 2 |
| Final body weight (g) | 206 ± 3 | 202 ± 5 | 202 ± 6 |
| Food intake (g/ day) | 18.7 ± 0.8 | 18.7 ± 0.9 | 18.5 ± 1.0 |
| Liver weight (g/100 g body weight) | 4.0 ± 0.1a | 5.6 ± 0.2b | 5.4 ± 0.3b |
| Rats were paired-fed orotic acid supplemented diets with/or without Shochu kasu or a diet without orotic acid (basal diet) for 10 days. Values are expressed as mean ± SEM of five rats. abIndicate significant differences at p<0.05 | |||
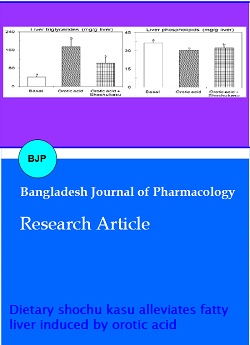
The liver triglyceride content of orotic acid-fed rats given dietary shochu kasu was decreased by 45% (p<0.05, Figure 1). The hepatic triglyceride content of orotic acid group involved 17.5% of the liver weight. However, the hepatic triglyceride contents of basal and shochu kasu-containing diets involved 0.5% and 10.2% of the liver weights, respectively (p<0.05). The liver phospholipids and cholesterol (data not shown) contents however were nearly same level in both groups containing orotic acid. The alleviation of fatty liver by dietary shochu kasu in orotic acid-fed rats was not associated with the reduction of serum lipids level (Figure 2) because serum lipid levels of shochu kasu group were slightly increased.
Figure 1: The effects of dietary shochu kasu on liver triacylglycerol and phospholipid content of OA-treated rats Rats were paired-fed OA supplemented diets with/or without shochu kasu or a diet without OA (basal diet) for 10 days. Values are expressed as mean ± SEM of five rats. abIndicate significant differences at p<0.05
Figure 2: The effects of dietary shochu kasu on serum lipid level of OA-treated rats Rats were paired-fed OA supplemented diets with/or without shochu kasu or a diet without OA (basal diet) for 10 days. Values are expressed as mean ± SEM of five rats. abIndicate significant differences at p<0.05
Dietary shochu kasu in orotic acid-fed rats attenuated the fatty acid synthase activity by 35% (p<0.05, Figure 3). Shochu kasu intake inhibited biosynthesis of fatty acids in orotic acid-treated rats. Dietary orotic acid- supplemented diet, however, stimulated the fatty acid biosynthesis. The reduction of fatty acid synthase activity by dietary shochu kasu in orotic acid-fed rats progressively attenuated the phosphatidate phospho-hydrolase activity; the phosphatidate phosphohydrolase level decreased by 20% (p<0.05).
Figure 3: The effects of dietary shochu kasu on lipogenic enzymes activity of OA-treated rats. Rats were paired-fed OA supplemented diets with/or without shochu kasu or a diet without OA (basal diet) for 10 days. Values are expressed as mean ± SEM of five rats. abIndicate significant differences at p<0.05
Discussion
Shochu kasu contains significant amounts of PUFAs mainly linoleic acid (Yamasaki et al., 2006). Dietary PUFA reduced fatty liver induced by orotic acid (Buang et al., 2004). This present study found that dietary shochu kasu reduced markedly the triglyceride accumulated in liver. The decrease of hepatic triglyceride was associated with the decrease in fatty acid synthase and phosphatidate phosphohydrolase activities. The reduction of these enzyme activities in rats with shochu kasu is the key factor of the alleviation of fatty liver induced by orotic acid.
Fatty liver is indicated by an excessive accumulation of triglyceride (more than 5-10%) in the liver (Sherlock and Dooley, 1997). High triglyceride content in the liver can result from two mechanisms: 1) impairment of secretion of very-low density lipoproteins (VLDL) from the liver, or 2) the increased expression of lipogenic enzymes and genes combined with the impaired entry of fatty acids into the mitochondrial P-oxidation pathway (Buang et al., 2005). To investigate the underlying mechanism by which dietary shochu kasu alleviated the orotic acid-induced triglyceride accumulation in the liver, the serum lipids level and hepatic enzyme activities involved in fatty acid and triglyceride biosyntheses were determined. Firstly, the serum lipid level was determined. Serum lipids level of orotic acid fed rat decreased significantly in comparison to the basal diet. However, there was not found the difference between the last two groups. The addition of shochu kasu in orotic acid-treated rats therefore did not affect the lipid secretion. Numerous studies have reported that dietary orotic acid reduced serum lipid levels (Cha et al., 1998; Buang et al., 2004; Buang et al., 2005). The mechanism by which orotic acid reduces serum lipid levels is due to interferes of the secretion of VLDLs (Pottenger and Getz, 1971). Those reports are consistent with results of serum lipid level in present study. Overall, the lowering triglyceride level in liver of orotic acid-treated rats given dietary shochu kasu was not caused by the impairment of VLDL secretion from the liver. Secondly, the lipogenic enzyme, the fatty acid synthase and phosphatidate phosphohydrolase, activities were determined. The fatty acid synthase of orotic acid group was higher than that of the basal group. This result is absolutely consistent with the previous studies (Buang et al., 2004; Buang et al., 2005; Cha et al., 1998). These data suggested that dietary orotic acid promoted concentration of the reduced forms of nicotinamide adenine dinucleotide phosphate (NADPH), a coenzyme of fatty acid synthase (Lehninger et al., 1993). This result is in agreement with the reports by Pottenger and Getz in 1971 that orotic acid did not interfere the protein biosynthesis in rat. The addition of shochu kasu into the orotic acid diet, however, attenuated the fatty acid synthase activity by 35%. The underlying mechanism presumably related to the competitive reactions involving NADPH for the two different pathways: 1) the fatty acid biosynthesis and 2) the fatty acid desaturation.
The fatty acid biosynthesis is catalyzed by fatty acid synthase, in which NADPH is used as the cofactor. The maximum rates of the enzyme need a maximum concentration of NADPH. The low concentration of NADPH in the metabolism systems can occur from two possible reasons: 1) its biosynthesis is decreased, or 2) a part of the NADPH moves into the other pathways, such as fatty acid desaturation, in which NADPH acts as a cofactor of fatty acyls desaturases. The last mechanism is possible because shochu kasu contains linoleic acid (Yamasaki et al., 2006).
In vitro study found that shochu kasu incubated with Schizochytrium sp. generates a significant amounts of docosahexaenoic acid (DHA) at maximum pH (pH 7.5) in presence of high glucose (Yamasaki et al., 2006). Shochu kasu also contain alpha-linolenic acid), the parent of DHA (the ©3-PUFA). The Schizochytrium sp. therefore might fertile with shochu kasu and be able to deliver the ©3-PUFA, DHA. The linoleic acid and DHA contained in shochu kasu that was intake into the orotic acid-treated rats could reduce the lipogenic enzyme activities of the rat liver. It was because both linoleic acid and DHA can reduce fatty acid synthase level as reported (Moon et al., 2002). The fatty acid synthase level decreased by the diet shifts NADPH into the fatty acid desaturation pathways and therefore this mechanism might promote PUFA level. The latter mechanism is reasonable to reduce the NADPH level provided for fatty acid biosynthesis pathway, and consequently fatty acid synthase activity decreased. The attenuation of fatty acid synthase activity in orotic acid-fed rats given dietary shochu kasu indicated that the fatty acyl-CoA concentration decreased. Fatty acyl-CoA is an activated fatty acid that is a general substrate of the other lipid biosyntheses includes liver triglyceride, phospholipids, cholesterol, etc. and the substrate for fatty acid β-oxidation (Lehninger et al., 1993).
The reduction of fatty acid synthase activity indicated the reduction of fatty acid generated. The decreased fatty acid synthase activity therefore is reasonable to reduce phosphatidate phosphohydrolase level. It is because one of the substrate of phosphatidate phosphohydrolase enzyme is fatty acyl-CoA derived from fatty acid. Phosphatidate phosphohydrolase is known as a rate-limiting enzyme of triglyceride biosynthesis (Walton et al., 1985; Cha et al., 1998). Phosphatidate phosphohydrolase hydrolyzes phosphatidic acid to generate diglyceride and thereafter fatty acyl-CoA esterifies diglyceride catalyzed by phosphatidate phosphohydrolase to generate triglyceride. Both fatty acyl-CoA and diglyceride are substrates of phosphatidate phosphohydrolase. The low level of fatty acyl-CoA causes esterification of diglyceride to form triglyceride, which decreases and therefore phosphatidate phosphohydrolase activity decreases. The reduction of phosphatidate phosphohydrolase activity in liver by dietary shochu kasu in orotic acid-treated rats attenuated the hepatic triglyceride accumulation. In addition, the level of fatty acid beta-oxidation in orotic acid-treated rats given dietary shochu kasu was similar between the orotic acid-containing groups (data not shown). It indicated that fatty acid degradation was not affected by the intake of shochu kasu in orotic acid-treated rats.
Conclusion
The reduction of liver triglyceride level in orotic acid-treated rats given dietary shochu kasu was not associated with the induction of fatty acid beta-oxidation and the promotion of serum lipid levels. Intake of shochu kasu inhibits the activities of fatty acid synthase and phosphatidate phosphohydrolase and consequently reduces triglyceride biosynthesis. Dietary shochu kasu therefore alleviates fatty liver induced by orotic acid.
Acknowledgements
The authors would like to appreciate the excellent assistance from Dr. Koji Nagao in enzymatic determinations and the useful assistance from Dr. Yu-Ming Wang in handling instruments and animals. The appreciation is also given to all of the members of Laboratory of Applied Biochemistry-Saga University. The authors also give thanks to the Japanese Monbukagakusho for providing the fund of the research.
References
Barlett GR. Phosphorous assay in column chromatography. J Biol Chem. 1959; 234: 466-68.
Buang Y, Wang YM, Cha JY, Nagao K, Yanagita T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition 2005; 21: 867-73.
Buang Y, Cha JY, Nagao K, Wang YM, Inoue N, Yanagita T. Alleviation of fatty liver by α-linolenic acid. J Nutr Sci Vitaminol. 2004; 50: 272-76. PMid: 15527069
Cha JY, Mameda Y, Yamamoto Y, Oogami K, Yanagita T. Association between hepatic triglycerol accumulation induced by administering orotic acid and enhanced phophatidate phosphohydrolase activity in rats. Biosci Biotechnol Biochem. 1998; 62: 508-13.
Cotrim HP, Parana R, Braga E, Lyra L. Nonalcoholic steatohepatitis and hepatocellular carcinoma: Natural history? Am J Gastroenterol. 2000; 95: 3018-19.
Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: Predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001; 121: 91-100.
Fletcher MJ. A colorimetric method for estimating serum triglycerides. Clin Chim Acta. 1968; 22: 393-97.
Folch J, Lees M, Sloane-stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957; 226: 497-509.
Harrison SA, Diehl AM. Fat and the liver: A molecular overview. Semin Gastrointest Dis. 2002; 13: 3-16.
Ikeda I, Cha JY, Yanagita T, Nakatani N, Oogami K, Imaizumi K, Yazawa K. Effect of dietary a-linolenic, eicosapentaenoic, and docosahexaenoic acids on hepatic lipogenesis and β-oxidation in rats. Biosci Biotechnol Biochem. 1998; 62: 675Â80.
Lehninger LA, Nelson DL, Cox MM. Principles of biochemistry. 2nd ed. USA, Worth Publishers, 1993.
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-75. PMid:14907713
Pottenger LA, Getz GS. Serum lipoprotein accumulation in the livers orotic acid-fed rats. J lipid Res. 1971; 12: 450-59.
Sherlock S, Dooley J. Diseases of the liver and biliary system. Oxford, Blackwell Science, 1997.
Starkel P, Sempaoux C, Leclercq I, Herin M, Debby C, Desager JP, Horsmans Y. Oxidative stress, KLF6 and transforming growth factor-β up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol. 2003; 39: 538-46.
Tsushin NK. The beverage and food statistics monthly. Tokyo, Nikkan Keizai Tsushin, 2005, p 133.
Walton PA, Possmayer F. Mg2-dependent phosphatidate phosphohydrolaseof rat lung: Development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal Biochem. 1985; 151:479-86.
Yamasaki T, Aki T, Shinozaki M, Taguchi M, Kawamoto S, Ono K. Utilization of shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J Biosc Bioeng. 2006; 102: 323-27.
Moon YS, Latasa MJ, Griffin MJ, Sul HS. Suppression of fatty acid synthase promoter by polyunsaturated fatty acids. J Lipid Res. 2002; 43: 691-98.
Yokoyama S, Hiramatsu J, Hayakawa K. Production of y-aminobutyric acid from alcohol distillery less by lactobacillus brevis IFO-12005. J Biosci Bioeng. 2002; 93: 95Â97.
Yokoyama S, Tarumi S. Production and some properties of low-salt seasoning from shochu distillery waste. Seibutsu-Kogaku 2001; 79: 211-17.