In vivo antioxidant and hepatoprotective effects of methanolic extract of dioclea reflexa seed in rats following acute or chronic liver injury
Abstract
Methanolic extract of Dioclea reflexa seed was investigated following acute or chronic carbon tetrachloride intoxication in male albino rats. In the acute experiment, rats were pre-treated with extract at 5 mg/kg for three days before administration of carbon tetrachloride (0.6 mL/kg) while in the chronic model, rats received extract at 2.5 mg/kg for 12 consecutive days with 72 hourly administration of carbon tetrachloride at 0.3 mL/kg. In both models, the levels of transaminases and malondialdehyde were significantly (p<0.05) reduced in D. reflexa extract treated rats compared to the carbon tetrachloride control, with concomitant boosting in the levels of superoxide dismutase and catalase activities. These results indicate that the seed extract of D. reflexa possess strong antioxidant and hepatoprotective properties in both acute and chronic hepatocellular damage and oxidative stress that it could impact positively on the health of the eastern Nigerian populace that consumes it regularly.
Introduction
It has been established that oxidative stress is among the major causative factors in the induction of many chronic and degenerative diseases including atherosclerosis, diabetes mellitus, cancer, Parkinson's disease, immune dysfunction as well as aging (Souri et al., 2008). Spices and herbs are recognized as sources of natural antioxidants that can protect man from oxidetive stress and thus play an important role in the chemoprevention against diseases that have their etiology and pathophysiology in reactive oxygen species (Atawodi, 2005; Rizwan et al., 2012; Vauzour et al., 2010). Thus, it has been recommended that adequate intake of antioxidant through the consumption of antioxidant rich foods can prevent the development of oxidative stress (Lako et al., 2008; Atawodi, 2012; Nisar et al., 2012). In furtherance of our earlier work on the antioxidant properties of Nigerian foodstuffs and herbs (Atawodi et al., 2009a; Atawodi et al., 2009b; Atawodi et al., 2010a; Atawodi, 2010; Atawodi et al., 2011a Atawodi, 2011b), the antioxidant and hepatoprotective activities of dried seeds of Dioclea reflexa, popular as condiment in Eastern Nigeria was investigated in acute and chronic models of liver injury and oxidative stress.
Dioclea reflexa Hook F. (Papilionaceae) ("Ukpo" in Igbo, "Agbaarin" in Yoruba) is an annual crop and a climber that can be cultivated more than once a year. The plant is high yielding, bears pod containing between three and four seeds from where it reproduces (Oladosu et al., 2006). The seeds are usually dark brown to black, sometimes speckled, depending on variety. Among the natives, the endosperm, which is rich in gum is pulverized and used as thickener in many traditional food preparations. Several authors have reported on its suitability for application in processed foods including use as a rheology modifier. Both its seed and leaf have high economic values in culinary and pharmaceutical industries (Agba et al., 2005).
Physicochemical analysis of the oil of D. reflexa seeds revealed the acid value, saponification value, iodine value, ester value and iodine number to be 8.69 mg KOH/g, 251 mg KOH/g, 72.8 mg I/g, 242 and 27.9, respectively. The fatty acid composition determined by gas chromatography (GC) showed individual unsaturated fatty acid to be oleic acid (18:1), 0.8%, while the saturated fatty acids were palmitic acid (16:0), 10.2% and stearic acid (18:0), 21.9%. The high saponification and iodine values of D. reflexa oil suggest its possible utilization in alkyd resin, shoe polish, liquid soap and shampoo production (Faleye, 2010). Both cake and seed flour of D. reflexa has appreciable levels of protein that could serve as important protein sources in livestock production, while its oil could be a good domestic and industrial substitute to the over increasing demands of conventional oils (Lasisi and Yusuf, 2006).
Materials and Methods
Chemicals and reagents: Methanol, petroleum ether, vitamin E as tocopherol acetate, carbon tetrachloride, corn oil, and assay kits were purchased from sigma chemical Co, Ltd (USA) and are of analytical grades.
Plant collection and authentication: D. reflexa seeds and plant parts were collected from Achalla village in Awka North Local Government of Anambra State Nigeria in June of 2009. It was authenticated at the Herbarium Unit of the Department of Biological Sciences, Ahmadu Bello University, Zaria, Nigeria where voucher number 1286 was assigned.
Sample preparation and extraction: The D. reflexa seeds were dried in the laboratory at room temperature and pulverized using laboratory mortar and pestle. Pulverized material (35 g) was placed in the thimble of soxhlex extractor and extracted first, using petroleum ether (300 mL) for 8 hours each and then methanol (300 mL), three times for 5 hours each. The methanol extracts were combined and dried in vacuo at 45°C using a rotary evaporator (Buchi Labortechnik AG, Switzerland). The methanol extracts were combined and dried in vacuo at 45°C using a rotary evaporator.
Experimental animals and management: Male albino rats (7-8 weeks old and weighing about 120-150 kg) were purchased from the animal house of National Research Institute for Chemical Technology, Zaria, Nigeria. They were acclimatized for two weeks prior to commencement of experiment. They were kept at room temperature and were maintained ad libitum on tap water and growers mash (Vita feeds, Jos, Plateau State Nigeria) except in the last 15 hours before termination of the experiment. They were weighed prior to commencement and termination of the experiment.
In vivo antioxidant activity of plant extract: In the chronic liver damage model, rats were divided into 7 groups of six rats each. Group 1 was administered the plant extract only, Group 2 was administered plant extract and carbon tetrachloride, Group 3 was administered solvent only, group 4 was administered solvent and carbon tetrachloride, Group 5 was administered vitamin E only, Group 6 was administered vitamin E and carbon tetrachloride, Group 7 was untreated control. The carbon tetrachloride was administered at a dose of 0.6 mL/kg body weight before the administration of the first extract or vitamin E, and subsequently every 72 hours until 10 days before termination of the experiment. After the first day, extract and vitamin E were administered daily at a dose of 2.5 mg/kg body weight for 10 days. The animals were sacrificed 24 hours after the last administration.
In the acute liver injury model, rats were divided into 7 groups with each group containing six rats. Group 1 was administered the plant extract only, Group 2 was administered plant extract and carbon tetrachloride, Group 3 was administered solvent only, Group 4 was administered solvent and carbon tetrachloride, Group 5 was administered vitamin E only, Group 6 was administered vitamin E and carbon tetrachloride and Group 7 was the untreated control. The rats received 24 hourly administration of the extract at 5 mg/kg for three days by intraperitoneal injection. On the third day, carbon tetrachloride (0.6 mL/kg body weight) was administered 1 hours after the extract administration, and the animals sacrificed 24 hours later (Atawodi et al., 2011a; Atawodi, 2011b; Lako et al., 2008).
Collection, storage and homogenization of tissues: Liver, kidney and heart of the experimental rats were collected after sacrifice, raised immediately in a cold normal saline (0.9% NaCl) and stored in ice. The tissues were homogenized after collection with phosphate buffer (pH 7.4) centrifuged at 3000 x g for 10 min. The clear supernatants were collected in Eppendorf tubes for in vivo assay. At the point of sacrifice, blood from each rat was withdrawn from carotid artery at the neck and collected in previously labeled test tubes and allowed to stand for 3 hours. Clear serum were collected from the blood in Eppendorf tubes and stored under -20°C for biological assay.
Determination of antioxidant status:
Catalase evaluation: Catalase activity was measured using a method (Abei, 1979). Exactly 10 µL of serum was added to test tube containing 2.8 mL of potassium phosphate buffer (50 mM; pH 7.0), and the reaction was initiated by the addition of 0.1 mL of freshly prepared hydrogen peroxide (30 mM), while the decomposition rate of H2O2 was measured spectrophotometrically at 240 nm after 5 min. A molar extincttion coefficient (E) of 0.041 mM/cm was used to calculate the catalase activity from the absorbance values obtained.
Superoxide dismutase estimation: Assay of superoxide dismutase activity was based on monitoring the superoxide dismutase-mediated decrease in the rate of autooxidation of hematoxylin in aqueous alkaline solution to yield a chromophore with maximum absorbance at 560 nm. This was carried out according to the method described elsewhere (Usha et al., 2007) using 920 µL of phosphate buffer (pH 7.8), 40 µL of sample and incubation for 2 min at 25°C before addition of hematoxylin was added (40 µL), quick mixing and reading the absorbance at 560 nm.
Determination of lipid peroxidation level: Lipid peroxidetion was determined as malondialdehyde as described elsewhere (Atawodi et al., 2011a; Atawodi, 2011b) using trichloroacetic acid and thiobarbituric acid. This was based on the principle that lipid peroxidation generates peroxide intermediates which upon cleavage release malondialdehyde, a product that reacts with thiobarbituric acid to form a colored complex which absorbs light at 535 nm. The method may be summarized as follows: Exactly 1 mL of 14%trichloroacetic acid was measured into a test followed with 1 mL of thiobarbituric acid and 50 µL of the tissue homogenate. The mixture was incubated at 80°C for 30 min in a water bath and allowed to cool rapidly under tap water before centrifugation 3000 x g for 10 min. The absorbance of the clear supernatant obtained was read spectrophotometrically at 535 nm.
Aspartate aminotransferase assay: Aspartate aminotransferase was determined as described elsewhere (Akram et al., 2006), using assay kits. Briefly the method involves formation of oxaloacetate hydrazone with 2,4-dinitrophenylhydrazine by reaction of 0.5 µL of reagent 1 (phosphate buffer, L-aspartate and alpha-oxoglutarate) with 0.1 mL of serum which was mixed and incubated for exactly 30 min at 37°C before 0.5 mL of reagent 2 (2,4-dinitrophenylhydrazine) was added, mixed and allowed to stand for exactly 20 min at 25°C. Following this, 0.4 M sodium hydroxide (0.5 mL) was added, mixed and absorbance was read against the reagent blank at 540 nm after 5 min. The aspartate transaminase concentration was estimated by extrapolation from the calibration curve of the standard.
Determination of alanine aminotransferase activity: Alanine aminotransferase was determined as described elsewhere (Akram et al., 2006), using assay kits. This involves exactly 0.5 µL of reagent 1 (phosphate buffer, L-alanine and α-oxoglutarate) which was added in a clean test tube containing 0.1 mL of serum, mixed and incubated for exactly 30 mun at 37°C before addition of 0.5 mL of reagent 2 (2,4-dinitrophenylhydrazine), mixing and allowing to stand for exactly 20 min at 25°C. The reaction was stopped with 0.5 mL of sodium hydroxide (0.4 M) and absorbance was read against the reagent blank at 540 nm after 5 min. The alanine transaminase concentration was determined by extrapolation from the calibration curve of the standard compound.
Results
Figures 1 presents the levels of aminotransferases in rats chronically or acutely intoxicated with carbon tetrachloride with or without D. reflexa seed extract pre-treatment. In both models, pre-treatment with the extract significantly reduced (p<0.05) the levels of alanine transaminase and aspartate transaminase when compared to the carbon tetrachloride control. However, the lowering effect of the extract was more pronounced in the model of chronic hepatotoxicity (Figure 1A) compared to the acute model (Figure 1B).
Figure 1: Mean aminotransferases activities in serum following daily intraperitoneal administration of D. reflexa extract (2.5 mg/kg) with 72 hourly injection of carbon tetrachloride (0.3 mL/kg) for 10 days (A); intoxicated with carbon tetrachloride (0.6 mL/kg) following three days pre-treatment with methanol extract of D. reflexa (5.0 mg/kg) (B)
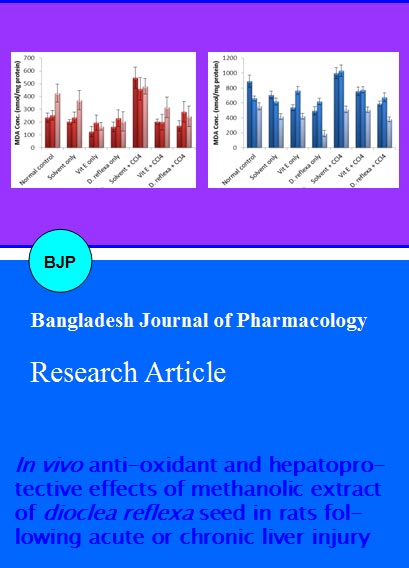
In the chronic experimental model, there was a significant (p<0.05) reduction in the level of malondialdehyde in the D. reflexa extract treated groups compared to the control group in all organ studied particularly the liver and kidney. However, no significant difference existed between the groups treated with methanol extract of D. reflexa seeds and vitamin E control for all organs (Figure 2A). Similar trend was observed in the model of acute liver damage where rats were pre-treated for three days with methanol extract of D. reflexa seeds before intoxication with carbon tetrachloride (Figure 2B).
Figure 2: Mean malondialdehyde levels in different tissues of rats following daily intraperitoneal administration of D. reflexa extract (2.5 mg/kg) with 72 hourly carbon tetrachloride intoxication (0.3 mL/kg) for 10 days (A); rats intoxicated with carbon tetrachloride (0.6 mL/kg) following three days pre-treatment with methanol extract of D. reflexa (5.0 mg/kg) (B)
Whereas no significant difference existed between the catalase levels in the vitamin E and extract control for most of the organs, pre-treatment with methanol extract of D. relexa significantly boosted the level of this enzyme when compared to the carbon tetrachloride control in both chronic (Figure 3A) and acute (Figure 3B) models of liver damage.
Figure 3: Mean levels of catalase in the serum, liver, kidney, and heart of rats following daily intraperitoneal administration of D. reflexa extract (2.5 mg/kg) with 72 hourly carbon tetrachloride intoxication (0.3 mL/kg) for 10 days (A); rats intoxicated with carbon tetrachloride (0.6 mL/kg) following three days pre-treatment with methanol extract of D. reflexa (5.0 mg/kg) (B)
The activity of superoxide dismutase in the serum, liver, kidney and heart homogenates following chronic and acute CCl4 induce liver injury with or without pre-treatment with methanol extract of D. reflexa seeds are presented in Figure 4, respectively. Like vitamin E, pre-treatment with methanol extract of D. reflexa seed significantly prevented the carbon tetrachloride induced depletion in the level of superoxide dismutase in both acute and chronic models of liver injury.
Figure 4: Mean serum, liver, kidney, and heart superoxide dismutase levels of rats following daily intraperitoneal administration of D. reflexa extract (2.5 mg/kg) with 72 hourly carbon tetrachloride intoxication (0.3 mL/kg) for 10 days (A); rats intoxicated with carbon tetrachloride (0.6 mL/kg) following three days pre-treatment with methanol extract of D. reflexa (5.0 mg/kg) (B)
Discussion
The levels of superoxide dismutase and catalase activities were significantly lower in the carbon tetrachloride control group than in the extract or vitamin E-treated groups. Also the carbon tetrachloride-induced significant elevation in the levels, aspartate aminotransferase and alanine aminotransferase, malondialdehyde was prevented by treatment with the methanol extract of D. reflexa seed or vitamin E.
This is not surprising since the hepatotoxic effects of carbon tetrachloride leads to formation of lipid peroxidase, which in turn gives products like malondialdehyde that cause damage to the membrane (Singh et al., 2011). That the extract administered groups showed significantly lower level of malondialdehyde in all the three organs compared to the carbon tetrachloride control group suggest that the membrane damage caused by carbon tetrachloride was prevented or ameliorated by extract pre-treatment or administration as decreased levels of malondialdehyde in extract-treated rats indicated that the extract could scavenge free radicals in the rat tissues.
The activities of catalase and superoxide dismutase which were tested in the serum, liver, kidney and heart of the experimental rats, showed a significant increase in all the organs and serum of the extract and vitamin E-treated groups over the carbon tetrachloride control group. It is known that superoxide dismutase is present in mitochondria while catalase is found principally in peroxisomes and to a lesser extent in the cytosol and microsomal fraction of the cell, the highest activities being found in tissues with a high peroxisomal content (kidney and liver) (Colleen, 2003). Thus, the lower activity in the carbon tetrachloride control rats suggests oxidative stress, as the rate of reactive oxygen species generation would exceed the capacity of the cell system to neutralize them due to the depleted level of the antioxidant enzyme system. This is because the conversion of superoxide anion to hydrogen peroxide and oxygen by superoxide dismutase, often called the primary defense mechanism against oxidative stress as superoxide is such a strong initiator of chain reactions, while hydrogen peroxide, once formed, must be reduced to water and molecular oxygen to prevent it from forming the hydroxyl radical by catalase that normally converts hydrogen peroxide to oxygen and water.
Pre-treatment with the methanol extract of D. reflexa significantly prevented the carbon tetrachloride mediated elevation in the levels of alanine transaminase and aspartate transaminase. Whereas significantly elevated levels of these serum marker enzymes suggest oxidative damage in the carbon tetrachloride control, as there levels showed good correlation with the severity of hepatic injury (Colleen, 2003), the significantly lower levels of these enzyme in the extract pre-treated group indicate a strong hepatoprotective potential when seeds of D. reflexa or products therefrom are consumed. Thus, the consumption of this popular condiment may have wide ranging implication in preventing and ameliorating oxidative stress related illnesses in the in the population where it is popularly consumed.
The ability of the D. reflexa seed methanol extract to protect and ameliorate the organs of the rat against oxidative stress-related damage should be as a result of its polyphenols and flavonoids content, since polyphenols, especially flavonoids have been reported to exert positive effects on human health through their antioxidant properties and antimutagenic properties (Norhaiza et al., 2009; Atawodi, 2012 ; Nisar et al., 2012; Rizwan et al., 2012; Vauzour et al., 2010). However, studies are underway to establish the specific polyphenols in D. reflexa seeds that are responsible for these desirable health effects.
Acknowledgement
The research work of SEA is partly supported through material donation from Alexander von Humboldt Foundation of Germany, and the assistance is hereby appreciated.
References
Abei H. Catalase. In: Method of enzymatic analysis. New York, Academic Press, 1979, pp 673-84.
Agba OA, Asiegbu JE, Omalik CPE. Effect of length of soakingin water at room temperature and hot water treatment on the germination of mucuna flagellipes (vogel Ex hook) seeds. Glob J Agric Sci. 2005; 4; 15-18.
Akram J, Mohammad JK, Zahra D, Hossein N. Hepatoprotective activity of Cichorium intybus L. leaves extract against carbon tetrachloride induced toxicity. Iran J Pharm Res. 2006; 1: 41-46.
Asuku O, Atawodi SE, Onyike E. Antioxidant, hepatoprotective, and ameliorative effects of methanolic extract of leaves of Grewia mollis Juss. on carbon tetrachloride treated albino rats. J Med Food. 2012; 15: 83-88.
Atawodi SE. Antioxidant potential of African medicinal plants. Afri J Biotechnol. 2005; 4: 128-33.
Atawodi SE, Atawodi JC, Idakwo P, Pfundstein B, Haubner R, Wurtele G, Spiegelhalder B, Bartsch HRW, Owen RW. Evaluation of the polyphenol composition and antioxidant activity of African variety of Dacryodes edulis (G.Don) H.J Lam fruit. J Med Food. 2009a; 12: 1321-25.
Atawodi SE, Atawodi JC, Pala Y, Idakwo P. Assessment of the polyphenol profile and antioxidant properties of leaves, stem and root barks of Khaya senegalensis Desv. Electron J Biol. 2009b; 5: 80-84.
Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B, Haubner R, Wurtele G, Spiegelhalder B, Bartsch HRW, Owen RW. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem and root barks of Moringa oleifera, Lam. J Med Food. 2010a; 13: 710-16.
Atawodi SE. Polyphenol composition and in vitro antioxidant potential of Nigerian Canarium schweinfurthii Engl. oil. Adv Biol Res. 2010; 4: 314-22.
Atawodi SE, Atawodi JC, Pfundstein B, Spiegelhalder B, Bartsch H, Owen R. Assessment of the polyphenol components and in vitro antioxidant properties of Syzygium aromaticum (L.) Merr. and Perry. Electron J Environ Agric Food Chem. 2011a; 10: 1970-78.
Atawodi SE. Nigerian foodstuffs with prostate cancer chemopreventive polyphenols. Proceedings, science of global prostate cancer disparities in black men conference. Infect Ag Can. 2011b; 6: S2-S9.
Atawodi SE. Antimutagenic polyphenols in African foods: Sixth annual conference on environmental mutagens in human population. Q Sci Proc. 2012; 3: 96.
Colleen S, Allan MD, Micheal L. Marks basic medical biochemistry: A clinical approach. 2nd ed. 2003, p 453.
Faleye FJ. Extraction and characterisation of Dioclea reflexa Hook F. seed oil. Pak J Sc Ind Res. 2010; 53: 40-48.
Lako J, Trenerry VC, Rochfort S. Routine analytical methods for use in South Pacific regional laboratories for determining naturally occurring antioxidants in food. Int Food Res J. 2008; 15: 1-11.
Lasisi AA. Yusuf AA. Compositional analysis of horse eye (Dioclea Reflexa) seed flour and its cake. Agric J. 2006; 10: 28-31.
Norhaiza M, Maziah M, Hakiman M. Antioxidative properties of leaf extracts of a popular Malaysian herb, Labisia pumila. J Med Plants Res. 2009; 3: 217-23.
Nisar M, Qayum M, Ćavar S, Shah MR, Zia-Ul-Haq M, Khan I, Ahmad KW, Qayum ZA. Chemical constituents and antioxidant activity of n-hexane extract of Impatiens bicolor Royle. Chem Nat Comp. 2012; 48: 143-46.
Oladosu IA, Echeme JO, Zubair MF. Anticholinesterase and antibacterial activities of dioclimidazole from Dioclea reflexa seeds. Fitoterapia 2006; 77: 571-75.
Rizwan K, Zubair M, Rasool N, Riaz M, Zia-Ul-Haq M, De Feo V. Chemical and biological study of Agave attenuata. Int J Mol Sci. 2012; 13: 6440-51.
Singh S, Mehta A, Mehta P. Hepatoprotective activity of Cajanus cajan against carbon tetrachloride induced liver damage. Int J Pharm Pharm Sci. 2011; 3: 146-47.
Souri E, Amin G, Farsam H, Barazandeh TM. Screening of antioxidant activity and phenolic content of 24 medicinal plant extracts. DARU. 2008; 16: 83-87.
Usha K, Mary KG, Hemalatha P. Hepatoprotective effect of hygrophila spinosa and cassia occidentalis on carbon tetrachloride induced liver damage in experimental rats. Indian J Clin Biochem. 2007; 22: 132-35.
Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrition 2010; 2: 1106-31.