Resibufogenin inhibits the growth of human osteosarcoma MG-63 cells via mitochondrial pathway
Abstract
Resibufogenin, a low molecular weight bufanolide steroid compound, is isolated from the secretion of Asiatic toad Bufogargarizansa Cantor. It possessed both pharmacological and toxicological effects that were experimentally shown by in vitro and in vivo studies. However, the molecular mechanism of cell apoptosis induced by resibufogenin remains elusive. Here, we investigated the apoptosis in resibufogenin-treated human osteosarcoma MG-63 cells. The results showed that resibufogenin could inhibit cell proliferation and induce apoptosis in a dose- and time-dependent manner. Additional investigations proved that a disruption of mitochondrial transmembrane potential and an up-regulation of reactive oxygen species (ROS) in resibufogenin-treated cells were occurred. Upon western blot analysis, it was found that the up-regulation of Apaf-1, cleaved PARP, cleaved caspase-3, -9, and Bax/Bcl-2, varied with different concentration of resibufogenin. Overall findings suggested that resibufogenin could be used as an effective anti-tumor agent in therapy of osteosarcoma.
Introduction
Osteosarcoma is a cancerous tumor that occurs in the bone. In China, the rate of osteosarcoma is 0.5 incidences per 100,000 individuals. The cancer accounts for about 51% of the incidence of childhood cancer, with the majority (50-70%) occur to adolescents of 10-20 years old. Generally, the symptoms are mild at early stages, but the disease is highly aggressive. As such, nearly 75% of the patients are diagnosed at a more advanced stage of cancer, i.e. enneking stage IIB or later. With modern surgical technology, the average five-year survival rate of osteosarcomais relatively high at around 66% (Aljubran et al., 2009). However, the survival rate drops to merely 25% with the occurrence of metastasis, primarily lung metastasis, which remains the most significant burden in osteosarcoma-related deaths (Gorlick et al., 2003; Ta et al., 2009).
Resibufogenin is an isolated compound of Chansu, a traditional Chinese medicine obtained from the skin venom gland of the toad. Dried toad venom is called "Chan su" or "toad cake" in China and "Senso" in Japan obtained from the postauricular and skin glands of toad. It is often found in traditional Chinese medicine ingredients, such as Liushen tablet (Hong et al., 1992)and Niuhuangxiaoyan tablet (Morishita et al., 1992). These Chinese medications have been widely used in China, Japan and other Asian countries for a long time, and over the last decade, have gained considerable favor in the United States and other places of the world. Toad venom is used as a topical anesthetic and cardiac medication (Zhang et al., 1991; Chow et al., 2003). It can eliminate toxic material; relieve carbuncle, furuncle, cellulites and multiple abscesses (Chen et al., 1967). Recently, toad venom has been utilized in the treatment of cancer (Groopman et al., 1992; Li, 1998; Yeh et al., 2002; Ye et al., 2003). However, overdose may cause nausea, vomiting, diarrhea, and even general paralysis (Xie et al., 2001). Recent reports indicate that toad venom toxicity carries a high mortality rate in the United States (Brubacher et al., 1999; Ko et al., 1996). Since, resibufogenin is a major isolated component from toad venom, the identification and determination of resibufogenin will play an important role in the safety, efficacy and therapeutic reproducibility of toad venom and its medical preparations.
Basically resibufogenin exhibits three major pharmacological effects that includes cardiotonic, vasopressor and respiratory stimulator. A number of animal experiments demonstrated that resibufogenin increased ventricular contractile force by 34% in rabbits, 36% in cats, and 32 or 50% in adult mongrel dogs (Dasgupta et al., 2000; Iwatsuki et al., 2000). Just as digitalis does resibufogenin increases the contractility of cardiac muscle in a dose dependent manner, a positive inotropic effect. This is why, Chansu formulations are a cardiotonic in clinic. Several reports have indicated that in the hemorrhaged animal model there was a significant increase in mean system arterial pressure following the administration of resibufogenin. The reason was thought to be due to an increase in cardiac output without a significant change in heart rates (Leigh et al., 1969). Uniquely, resibufogenin was demonstrated to be an efficacious respiratory stimulator. Unfortunately, no studies have been carried on the inhibitory effect of resibufogenin on OS and its mechanisms of the anticancer capacity. Therefore, the aim of the present study was to thoroughly research resibufogenin-induced apoptosis and explore the potential mechanisms.
Materials and Methods
Reagents and antibodies
Resibufogenin was purchased from Sigma-Aldrich, and its structure was shown in (Figure 1). It was dissolved in cell culture medium at a stock concentration of 20 mg/mL and stored at -20°C. Resibufogenin stock solution was freshly diluted in the medium just before the use in each experiment. Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kit (Becton Dickinson, Franklin Lakes, NJ), Hoechst-propidium iodide (PI) staining assay kit (Molecular Probes, Beyotime Institute of Biotechnology, Shanghai, China), 2,7-dichlorodihydrofluorescein diace-tate (DCFHDA; Beyotime Institute of Biotechnology, Shanghai, China); bcl-2, bax, anti-caspase-9, anti-caspase-7, anti-caspase-3, and beta-actin(anti-human) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were the other main substances that were utilized in biological experiments.
Figure 1: The chemical structure of resibufogenin
Cell lines and culture methods
The human OS MG-63 cells were maintained in RPMI-1640 supplemented with 10%fetal calf serum (heat inactivated, FCS), 2 mM glutamine, penicillin (100 U/mL), and streptomycin (100 mg/mL) at 37°C with 5%CO2. The cells were kept in an exponential growth phase during experiments.
Proliferation test by MTT assay
Cell growth inhibition by resibufogenin was analyzed by the 3-(4,5-dimethyl-thiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay. In this experiment, MG-63 cells were seeded in 96-well plates at a density of 6 x 103 cells per well. After treatment with various concentrations of resibufogenin (0.0-40.0 nM) for 24, 48, and 72 hours, 20 uL MTT (5 mg/mL) was added. After 4 hours, 100 uL DMSO was added and the formazan crystals resulted were dissolved. Absorbance was read at 490 nm using an enzyme-linked immunosorbent assay reader (SpectraMax; Molecular Devices, USA). Data were collected from three separate experiments, and the percentage of resibufogenin-induced cell growth inhibition was determined by comparison to DMSO-treated control cells.
Annexin V-FITC/PI double staining for apoptosis
Annexin V-FITC/PI double staining was employed to quantify the apoptosis of human OS cells treated with resibufogenin. Briefly, cells were seeded in 6-well plates at a density of 2 x 105 cells per well and exposed to resibufogenin (0.0-40.0 nM) for 24 hours. The cells were then stained using Annexin V-FITC/PI double-Xuorescence apoptosis detection kit (Biouniquer Technology, China) following the manufacturer's instruction. Samples were analyzed using a FACS CaliburXow cytometer within 1 hour after the staining. Cells were grown in 6-well plates for 12 hours and treated with caspase inhabitor Z-VAD-FMK (5 mM) for 1 hour before treated with resibufogenin. After 24 hours, cells were washed twice with PBS, adjusted to 100 uL of the solution, and transferred to a 1 mL centrifuge tube (1 x 105 cells). Ten microliter of Annexin V-FITC and 10 uL of PI were added, and cells were incubated for 15 min at room temperature (25°C) in the dark before being analyzed as described above.
Measurement of mitochondrial transmembrane potential with fluorescent JC-1
Cultured cells were resuspended with culture medium to a concentration of 1 x 105 cells/well and incubated with fluorescent dye JC-1 (20 nM) at 37°C for 30 min in the dark and then the fluorescent dye JC-1 fluorescence was immediately analyzed with a flow cytometer (Becton Dickinson, USA).
Intracellular ROS levels measurement
The intracellular accumulation of reactive oxygen species (ROS) in the cells was assessed using 20,70-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime Institute of Biotechnology, Shanghai, China). This nonfluorescent compound accumulates within cells, and on deacetylation, DCFH-DA then reacts with ROS and leads to the formation of fluorescent dichlorofluorescein (DCF). In this experiment, cells were seeded in 6-well plates at a density of 2 x 105 cells per well and exposed to 0.0-40.0 nM range of resibufogenin for 24 hours. Then it was washed twice with PBS and incubated with DCFH-DA (20 uM) in PBS for 1 hour at 37°C in the dark. Later, cells were then washed three times with PBS and finally examined in PBS supplemented with 10%fetal calf serum using a flow cytometer (Becton-Dickinson).
Immunoblotting
Cells were separately washed, collected, and homogenized in a lysis buffer (10 mM Tris-HCl, pH 8, 0.32 mM sucrose, 5 mM EDTA, 2 mM dithiothreitol, 1 mM phenyl methyl sulfonylfluoride, and 1%Triton X-100), and centrifuged (13.000 x g, 10 min, 4°C). To ensure that an equal amount of protein was loaded in each case, western blots were also carried out, using the Bradford protein assay. Equal amounts of proteins (50 μg) were subjected to electrophoresis in a sodium dodecyl sulfate-polyacrylamide gel (10%). The gel-separated proteins were transferred to nitropure nitrocellulose membranes (Santa Cruz Biotechnology), and the membranes were blocked with 10%bovine serum albumin in TBST [10 mM Tris-HCl (pH 8.0), 137 mM NaCl, and 0.05% Tween-20 by vol] overnight at 4°C and probed with primary antibodies at 37°C for 2 hours. Each of the targeted proteins was immuno-stained by specific antibodies. The antibodies used were anticleaved caspase-9, anti-cleaved caspase-7, anti-cleaved caspase-3, anti-cleaved PARP, anti-Bcl-2, anti-Bax, and anti-beta-actin at a dilution of 1:500, 1:500, 1:500, 1:500, 1:200, 1:200, and 1:1,000, respectively. The membranes were washed three times with TBST and then incubated for 1 hour at RT with alkaline phosphatase-conjugated secondary antibodies (Santa Cruz Biotechnology) before being visualized by using a chemiluminescence detection kit (Beyotime Institute of Biotechnology, Shanghai, China).
Statistical analysis
Data from triplicate experiments were analyzed with Student's t-test. Twoother independent experiments were performed, which gave identical results (statistically significant differences). A p value of <0.05 was considered to be statistically significant.
Results
To investigate the effects of resibufogenin on the proliferation of OS cells, we measured the growth of human OS cell lines using the MTT incorporation assay. MG-63 cells were treated with 0.0-40.0 nM of resibufogenin in a dose- and time-dependent manner. Experimental results showed in Table I revealed that resibufogenin inhibited the proliferation of human osteosarcoma cells in a dose- and time-dependent manner.
Table I:The proliferation of MG-63 cells inhibited by resibufogenin
| Group | 24 hours | 48 hours | 72 hours |
|---|---|---|---|
| Control | 0.402 ± 0.005 | 0.404 ± 0.005 | 0.405 ± 0.008 |
| 10 nM | 0.320 ± 0.003a | 0.310 ± 0.005a | 0.230 ± 0.003a |
| 20 nM | 0.295 ± 0.002b | 0.248 ± 0.006a | 0.199 ± 0.005b |
| 40 nM | 0.246 ± 0.003b | 0.212 ± 0.004b | 0.150 ± 0.004b |
| Results of MTT assay when MG-63 cells were treated with or without different concentrations of resibufogenin. Data are presented as means ± standard deviation from three independent experiments. Each experiment was conducted in triplicate, ap<0.05; bp<0.01 | |||
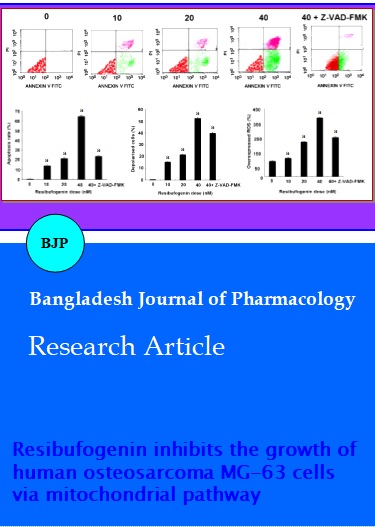
Resibufogenin induced the apoptosis of osteosarcoma cells. Flow cytometry assays showed marked changes in cell profiles after treatment with 0.0-40.0 nM resibufogenin, which strongly indicated that resibufogenin could induce apoptosis (Figure 2A). All resibufogenin treatment groups showed significant increases in apoptosiscompared with control groups (p<0.01). Apoptotic rates were ranged from 0.6 ± 0.1% to 67.4 ± 1.4% (Figure 2B). The 40.0 nM resibufogenin group was the highest in all experiments when compared with that of control group, p<0.01. Further, when added caspase inhibitor Z-VAD-FMK (5 mM) before exposure to 40.0 nM resibufogenin for 24 hours, apoptotic rates decreased to 24.2 ± 0.6% (p<0.01) (Figure 2B).
Cells treated with 10.0 nM resibufogenin showed a 16.3 ± 1.5% decrease in MMP (p<0.01). Cells treated with 20.0 nM resibufogenin showed a 23.7 ± 1.3% decrease in MMP (p<0.01). Finally, cells treated with 40.0 nM resibufogenin showed a 55.3 ± 1.7% decrease in MMP (p<0.01). All these results showed that mitochondrial transmembrane potential (MMP) was decreased in response to resibufogenin in a dose-dependent manner (Figure 2C). In addition, when added caspase inhibitor Z-VAD-FMK (5 mM) before exposure to 40.0 nM resibufogenin for 24 hours, MMP rates decreased to 38.12 ± 0.39 % (p<0.01) (Figure 2C).
Resibufogenin-induced intracellular ROS generation was evaluated using intracellular peroxide-dependent oxidation of DCFHDA to form fluorescent DCF. The group without DCFH-DA addition was served as a negative control (N-con). Results showed that ROS production in cells was significantly increased upon resibufogenin treatment groups when compared with that of the control (p<0.01) (Figure 2D).
Figure 2: The effects of resibufogenin on MG-63 cells. A: Representative photomicrographs of MG-63 cells stained with annexin V-FITC/PI after exposure of the cells to 0-40 nM resibufogenin for 24 hours. B: Flow cytometric analysis of MG-63 apoptotic cells stained for annexin V + propidium iodide (PI) after treatment with 0-40 nM resibufogenin, 40 nM resibufogenin +ZVAD- FMK. C: Mitochondrial membrane hyperpolarization induced by resibufogenin in MG-63 cells. Cells treated with various concentrations of resibufogenin for 24 hours followed by staining with fluorescent dye JC-1 and incubated at 37°C for 30 min in the dark. The mean fluorescence intensity was detected using a flow cytometer. The differences in the MMP levels between each group were expressed as a percentage of the control. D: Cells treated with various concentrations of resibufogenin for 24 hours were washed twice with PBS and incubated with DCFH-DA (20 mM) in PBS for 1 hour at 37°C in the dark. The level of intracellular peroxide was detected by DCFH-DA assay using flow cytometric analysis. ROS production in the MG-63 cells treated with various concentrations of Resibufogenin was significantly increased compared with control. Data are presented as means ± SD of three independent experiments, and each experiment was carried out in triplicate (ap<0.01)
To determine the mechanism responsible for resibufogenin mediated apoptosis, the apoptotic protein expressions were evaluated by Western blot analyses. Being PARP specific proteolytic cleavage by caspases is considered to be characteristic of apoptosis, the cleavage of PARP and caspases were evaluated. The results of Western blot analysis were shown in Figure 3 for cleaved PARP, cleaved capase-9, -7, -3, Bcl-2, and Bax proteins relative to beta-actin in control untreated cultures. Cultures exposed to 0.0-40.0 nM resibufogenin were also shown in the same figure. It can be seen that the exposure of cultures to resibufogenin resulted in up-regulation of cleaved PARP, cleaved capase-9, -7, -3, and Baxproteins that are all involved in apoptosis. In contrast, a decrease in the expression of Bcl-2 protein was also observed whenexposed to different concentrations of resibufogenin (Figure 3).
Figure 3: Western blot analysis of MG-63 cells after being exposed to 0-40 nM resibufogenin for 24 hours. Resibufogenin caused an up-regulation in the levels of Apaf-1, cleaved PARP, cleaved caspase-9, -7, -3, and down-regulation of Bcl-2/Bax, and each experiment was carried out in triplicate
Discussion
The results of the present study showed that MG-63 cells growth was inhibited by resibufogenin at different concentrations, ranging from 0 to 40 nM, for a period of about 24, 48, and 72 hours. Resibufogenin significantly decreased the proliferation of MG-63 cells in a dose and time-dependent manner. The apoptosis induced by resibufogenin was also found in a concentration-dependent manner through Hoechst-PI staining fluorescence imaging and flow cytometry.
In mammalian cells, apoptosis has been divided into two major pathways: The extrinsic pathway, activated by pro-apoptotic receptor signals at the cellular surface, and the intrinsic pathway regulates apoptotic cascades by the signaling convergence in the mitochondrion, which results in the alteration of the MMP, the release of cytochrome C into the cytosol, and the activation of caspase-9 (Hirsch et al., 1997). Independently from cell type and apoptosis inducer that MMP disruption is a constant feature of the apoptotic effector phase (Iannolo et al., 2008). Disruption of MMP and the subsequent release of apoptosis-promoting factors are considered key cellular events that trigger the intrinsic apoptotic pathway. Results from the present study clearly demonstrated that MG-63 cells treated with resibufogenin exhibited an early reduction of MMP, suggesting that MMP might play critical roles in resibufogenin-induced apoptosis. Similar to the role of mitochondria in the control of cell death, Bcl-2, and Bax, survival or apoptotic factors can also prevent or facilitate the release of apoptogenic factors such as cytochrome C (Mohamad et al., 2005; Orrenius et al., 2007; Yin et al., 2000). The loss of the Bcl-2 protein promotes the opening of the mitochondrial permeability transition pore. A decrease expression of Bcl-2 and an increase expression of Bax were observed in MG-63 cells after treatment with different concentrations of resibufogenin. This change in Bcl-2 and Bax expression may be enough to facilitate pore opening. Disruption of MMP is an early event in mitochondrial mediated apoptosis (Takahashi et al., 2004). After the reduction of membrane potential, a critical step is the formation of apoptosomes, which ultimately cleave procaspase-3 to form active caspase-3. Caspases play critical roles in the execution of apoptosis (Li et al., 2000). The results of the present study demonstrated that resibufogenin-induced apoptosis in MG-63 cells was mediated by caspase-9, -7, and -3 following PARP cleavage. Furthermore, the role that the caspases played in resibufogenin-induced apoptosis was confirmed by the attenuation of apoptosis in cells that were pretreated with Z-VAD-FMK.
Conclusion
Resibufogenin induced MG-63 cells apoptosis through mitochondrial permeability transition pore and caspase activation. Further, down-regulation of Bcl-2/Bax sustained caspase in the active state and triggered the mitochondrial pathway. All these findings provided a basis for the use of resibufogenin as a potential candidate in the treatment of osteosarcoma.
References
Aljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: Survival analysis with and without lung metastases. Ann Oncol. 2009; 20: 1136-41.
Brubacher JR, Lachmanen D, Ravikumar PR, Hoffman RS. Efficacy of digoxin specific Fab fragments (Digibind) in the treatment of toad venom poisoning. Toxicon 1999; 37: 931-42.
Chen KK, Korarikova A. Pharmacology and toxicology of toad venom. J Pharm Sci. 1967; 56: 1535-41.
Chow L, Johnson M, Wells A, Dasgupta A. Effect of the traditional Chinese medicines. Chan Su, Lu-Shen-Wan, Dan Shen, and Asian ginseng on serum digoxin measurement by Tinaquant (Roche) and Synchron LX system (Beckman) digoxin immunoassays. J Clin Lab Anal. 2003; 17: 22-27.
Dasgupta A, Biddle DA, Wells A, Datta P. Positive and negative interference of the Chinese medicine Chan Su in serum digoxin measurement: Elimination of interference by using a monoclonal chemiluminescent digoxin assay or monitoring free digoxin concentration. Am J Clin Pathol. 2000; 114: 174-79.
Gorlick R, Anderson P, Andrulis I. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: Meeting summary. Clin Cancer Res. 2003; 9: 5442-53.
Groopman JD, Zhu ZQ, Donahue PR, Pikul A, Zhang LS, Chen JS, Wogan GN. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi Autonomous Region, People's Republic of China. Cancer Res. 1992; 52: 4634-41.
Hong Z, Chen K, Yeung HW. Simultaneous determination of bufadienolides in the Traditional Chinese Medicine preparation, Liu-Shen-Wan, by liquid chromatography. J Pharm Pharmacol. 1992; 44: 1023-26.
Hirsch T, Marzo I, Kroemer G. Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep. 1997; 17: 67-76.
Iannolo G, Conticello C, Memeo L, DeMaria R. Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 2008; 66: 42-51.
Iwatsuki K, Yusa T, Kataoka Y, Sate K. Experimental and clinical studies of resibufogenin. Tohoku J Exp Med. 1965; 86: 93-101.
Ko RJ, Greenwald MS, Louscutoff SM, Au AM, Apple BR. Kreutzer RA, Haddon WF, Jackson TY, Boo FO, Presicek G. Lethal ingestion of Chinese herbal tea containing ch'ansu. West J Med. 1996; 164: 71-75.
Leigh JM, Caldwell AD. Some effects of RBG: An aglycone of animal origin. J Pharmacol. 1969; 21: 707-09.
Li ZQ. Traditional Chinese medicine for primary liver cancer. World J Gastroenterol. 1998; 4: 360-64.
Li M, Kondo T, Zhao QL, Li FJ, Tanabe K, Arai Y, Zhou ZC, Kasuya M. Apoptosis induced by cadmium in human lymphoma U937 cells through Ca2+-calpain and caspase-mitochondria dependent pathways. J Biol Chem. 2000; 275: 39702-09.
Mohamad N, Gutierrez A, Nunez M, Cocca C, MartÃn G, Cricco G, Medina V, Rivera E, Bergoc R. Mitochondrial apoptotic pathways. Biocell. 2005; 29: 149-61.
Morishita S, Shoji M, Oguni Y, Ito C, Higuchi M, Sakanashi M. Pharmacological actions of "kyushin," a drug containing toad venom: Cardiotonic and arrhythmogenic effects, and excitatory effect on respiration. Am J Chin Med. 1992; 20: 245-56.
Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: Implications forcell death. Annu Rev Pharmacol Toxicol. 2007; 47: 143-83.
Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: State of the art. Cancer Metast Rev. 2009; 28: 247-63.
Takahashi A, Masuda A, Sun M, Centonze VE, Herman B. Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm). Brain Res Bull. 2004; 62: 497-504.
Xie JT, Dey L, Wu JA, Lowell TK, Yuan CS. Cardiac toxicity of resibufogenin: Electrophysiological evidence. Acta Pharmacol Sin. 2001; 22: 289-97.
Yeh JY, Huang WJ, Kan SF, Wang PS. Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate 2002; 54: 112-24.
Ye M, Ning L, Zhan J, Guo H, Guo D. Biotransformation of cinobufagin by cell suspension cultures of Catharanthus roseus and Platycodon grandiflorum. J Mol Catal B: Enzymatic. 2003; 22: 89-95.
Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000; 10: 161-67.
Zhang LS, Nakaya K, Yoshida T, Kuroiwa Y. Bufalin as a potent inducer of differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1991; 178: 686-93.