Tubeimoside-1 up-regulates p21 expression and induces apoptosis and G2/M phase cell cycle arrest in human bladder cancer T24 cells
Abstract
Tubeimoside-1 (TBMS1) is a triterpenoid saponin with potent anticancer properties. In this study, for the first, we examined the antiproliferative effects of TBMS1 in human bladder cancer T24 cells and its ability to induce apoptosis and cell cycle arrest. Our results demonstrated that TBMS1 decreased the cell viability of bladder cancer T24 cells in a dose-dependent manner. Flow cytometric analysis showed that TBMS1 significantly triggered apoptosis in T24 cells and arrested cell cycle at G2/M phase in a dose-dependent manner. Further characterization demonstrated that TBMS1-induced apoptosis is associated with dissipation in mitochondrial membrane potential (ΔΨm), down-regulation of Bcl-2, and up-regulation of Bax and p21 in TBMS1-treated T24 cells. These in vitro results suggested that TBMS1 is an effective anti-bladder cancer natural compound that worth further mechanistic and therapeutic studies in human bladder cancer.
Introduction
Human bladder cancer is a potentially lethal and aggressive tumor has continuously increasing incidence all over the world (Ploeg et al., 2009). In the past decades, several natural compounds have shown a great promise in treatment of cancer and numerous natural compounds of dietary and botanical origin has been identified with chemotherapeutic and chemopreventive potential (Amin et al., 2009; Kintzios and Barberaki, 2004). Plants-derived natural compounds possess a variety of biological activities and draw considerable attention in pharmacological research due to their anti-inflammatory, antimalarial, antihepatitis, and anticancer activities (Khan et al., 2012; Lee et al., 2003; Tan et al., 1998). As novel therapeutic agents and treatment approaches are desired to improve the clinical outcome.
Triterpenoid saponins possess a variety of biological activities and play a significant role in pharmacological research due to their antifungal, antivirus, and anti-inflammatory activities (Magadula and Erasto, 2009; Woldemichael and Wink, 2001). Tubeimoside-1 (TBMS1) is a triterpenoid saponin, which possesses a variety of pharmacological functions, including anti-inflammatory, antitumorigenic, and anti-HIV activities (Yu et al., 1992; Yu et al., 1994). Furthermore, TBMS1 has antiproliferative effect and induced apoptosis in cancer cells such as lung cancer (Zhang et al., 2011), cervical cancer (Xu et al., 2009), nasopharyngeal carcinoma (Ma et al., 2008; Weng et al., 2003), melanoma A375 cells (Rasul et al., 2012b), and leukemia HL-60 cells (Yu et al., 1996). However, none of the previously published reports have shown its cytotoxic activity against human bladder cancer cells. Therefore, this study is the first to investigate the antiproliferative effect of TBMS1 in human bladder cancer T24 cells. Further characterization showed that TBMS1 effectively inhibited the proliferation of T24 cells through arresting cell cycle at G2/M phase and induction of apoptosis which is regulated by down-regulation of Bcl-2 and up regulation of Bax and p21. This study will pave the way for further in vivo studies of this potent anti-bladder cancer compounds.
Materials and Methods
Chemicals and reagents
Cell culture medium reagents and MTT [3'-(4,5 dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide], propidium iodide (PI), and dimethyl sulfoxide (DMSO) were purchased from Sigma. Fetal bovine serum (FBS) was purchased from the Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. Annexin V-FITC apoptosis detection kit was purchased from Beyotime Institute of Biotechnology Shanghai, China. Rabbit polyclonal anti-human Bcl-2, Bax, and p21 antibodies were purchased from Wuhan Boster Biological Technology Co., Ltd. beta-Actin, anti- mouse and anti-rabbit antibodies were purchased from Santa Cruz Biotechnology. Ponceou and cell lysis buffer for Western and IP were purchased from Bio SS Beijing. Rhodamine 123 was purchased from Eugene Co. (Oregon, USA).
Cell culture
Human bladder cancer cell line (T24) was purchased from the Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences (China). The cells were cultured in DMEM supplemented with 10% FBS and 100 unit of penicillin at 37°C in a CO2 incubator with 5% CO2, 95% air and 100% humidity. Cells were plated in 10 cm culture dish and allowed to grow to approximately 70% confluence before experimentation.
Cell proliferation assay
The growth inhibitory effect of the TBMS1 on the viability of cells was determined by the MTT assay. Briefly, T24 cells were plated at a density of 1 x 104 cells per well in 96-well plates. After 20 hours, cells were treated with 100 µL of complete culture medium containing 3, 6, 12.5, 25, 50 and 100 µM TBMS1 with equal amount of DMSO as negative control. Each concentration of TBMS1 was repeated in four wells. After incubation for 24 hours, cell viability was determined. 10 µL MTT (5 mg/mL) in phosphate buffered saline was added to each well and incubated for 4 hours. After removal of the medium, 150 µL DMSO was added to each well and shaken carefully. The absorbance was recorded on the microplate reader (ELX 800, BIO-TEK Instruments Inc.) at a wavelength of 570 nm. The inhibitory effect of TBMS1 on cell growth was assessed and inhibition ratio (I%) was calculated.
Determination of apoptosis
The apoptotic rate of T24 cells was detected using flow cytometry with the annexin V-FITC/PI double labeling method. Briefly, T24 cells in logarithmic growth phase were plated in 6-well plates and allowed to attach overnight. Cells were treated with 10 and 20 µM of TBMS1 for 24 hours. Then cells were collected, washed and resuspended in PBS. Apoptotic cell death was identified by double survival staining with recombinant FITC (fluorescent isothiocyanate)-conjugated annexin V and PI, using the annexin V-FITC apoptosis detection kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer's instructions. Flow cytometric analysis was performed immediately after staining. Data acquisition and analysis were performed in flow cytometry using Cell Quest software.
Cell cycle analysis
T24 cells were seeded in 12-well plates and then treated with 10 and 20 µM of TBMS1 for 24 hours. After treatments, the percentages of cells in the different phases of cell cycle were evaluated by determining the DNA content after propidium iodide staining as described somewhere else (Rasul et al., 2012a). Briefly, cells were washed with PBS, trypsinized and centrifuged at 1,000 rpm at 4°C for 5 min. Pellets were fixed overnight in 70% chilled ethanol. After fixation, cells were washed twice with PBS and incubated in PBS containing 10 µL/100 mL RNase (1 mg/mL) for 10 min at room temperature. Finally, samples were stained with 5 µL/100 mL of propidium iodide (1 mg/mL) for 30 min at 4°C. Data acquisition was done by flow cytometry (EPICSXL-MCL, Beckman Coulter, USA) using Cell Quest software.
Flow cytometric determination of mitochondrial membrane potential (ΔΨm)
To probe the changes in ΔΨm, T24 cells were stained with rhodamine-123 (1 µM) after treatment with 10 and 20 µM of TBMS1 for 24 hours with control group. The fluorescence of rhodamine-123 was measured by flow cytometry with excitation and emission wavelengths of 488 and 530 nm.
Western blotting
To reveal the mechanism of the apoptotic effect of TBMS1, Western blotting was performed for apoptotic related proteins as previously described (Rasul et al., 2012a). Briefly, T24 cells were incubated with 10 and 20 µM of TBMS1 for indicated time. Cells were trypsinized, collected in 1.5 mL centrifuge tube and washed with PBS. The cell pellets were resuspended in lysis buffer and were lysed on ice for 30 min. After centrifugation for 15 min, the supernatant fluids were collected and the protein content of the supernatant was measured by the NanoDrop 1000 spectrophotometer (Thermo Scientific, USA). The protein lysates were separated by electrophoresis on 12% SDS-polyacrylamide gel and transferred to a PVDF membrane (Amersham Biosciences, USA). The membranes were soaked in blocking buffer (5% skimmed milk) for 2 hours. To probe for Bcl-2, Bax, p21, and beta-actin; membranes were incubated overnight at 4°C with relevant antibodies, followed by appropriate HRP conjugated secondary antibodies and ECL detection.
Transient transfection and luciferase assays
Transient transfections were carried out using Lipofectamine 2000TM (Invitrogen) following the manufacturer's protocol. Cells were seeded into 48-well plates for 16 hours and co-transfected with reporter plasmid, p21WAF1/Cip1 promoter reporters, p21P-luc, (100 ng) in the presence of renilla luciferase control pREP7 vector 25 ng and then treated with TBMS1 (0, 5, 10, and 20 uM for 8 hours. Firefly luciferase activities were measured by using the Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis of data
For the statistical analysis of data, comparisons between results from different groups were analyzed with SPSS for Window Version 15.0. Student's t-test was employed to determine the statistical significance of the difference between different experimental groups and control group. p<0.05 value was defined as statistically significant. All experiments were repeated at least three times. Data were presented as mean ± standard deviation (SD).
Result and Discussion
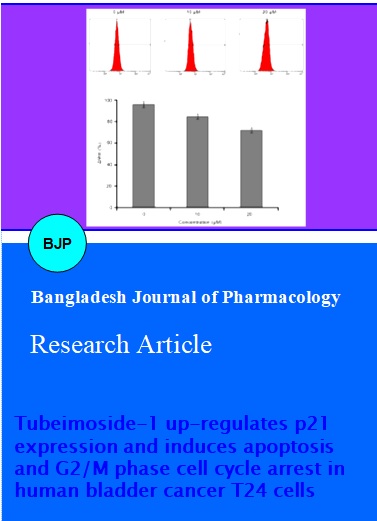
The investigation was initiated with screening of natural compounds against T24 bladder cancer cells. Human bladder cancer T24 cells were treated with different concentrations of TBMS1 (3, 6, 12.5, 25, 50 and 100 µM) for 24 hours. We found that TBMS1 exhibited cytotoxic effects on the propagation of T24 cells. TBMS1 is a natural compound that belongs to a triterpenoid saponin family. The structure of TBMS1 is shown in Figure 1. We determined the effects of TBMS1 on the viability of bladder cancer T24 cells by using MTT assay. TBMS1 showed antiproliferative effects against T24 cells in a concentration-dependent manner (Figure 2). Morphological changes were observed under phase contrast microscopy after treating cells with 10 and 20 µM TBMS1. The number of cells was significantly decreased and the cells became round-shaped (date not shown). The results indicated that TBMS1 induced growth inhibition of T24 cells and are consistent with the previously reported ones such as A-549 lung cancer cells (Zhang et al., 2011), HeLa cervical cancer cells (Xu et al., 2009), and nasopharyngeal carcinoma CNE-2Z cells (Ma et al., 2008; Weng et al., 2003). Our study is the first report of the anti-cytotoxic activity by TBMS1 against human bladder cancer cells.
Figure 1: Structure of tubeimoside-1 (TBMS1)
Figure 2: TBMS1 inhibited the cell proliferation and induced cell death. T24 cells were treated with indicated doses of TBMS1 for 24 hours and cell viability was measured by using MTT assay. The effect of TBMS1 on the cell growth inhibition of T24 cells compared with control group. The graph was plotted against mean values of percentages of three independent experiments. Data are expressed as mean ± SD (n = 3)
Recent insights related to cell cycle regulation indicated that there are a number of mechanisms which control the cell cycle to ensure the correct cell division. It is well known that progression of cell cycle is maintained by different check points in normal cells and the transition from one cell cycle phase to another occurs in an orderly fashion. In cancerous cells, some basic alterations transpired in the genetic control of cell division, resulting in a hyper cell proliferation. As the deregulation of cell cycle progression is the hallmark of cancer; thereby cell cycle regulation could be a potential and effective strategy for the treatment of cancer (Grana and Reddy, 1995; Vermeulen et al., 2003). Previous studies have reported that natural compounds have the potential to arrest cell cycle in a variety of cancer cells (Khan et al., 2011; Khan et al., 2012). Flow cytometry was performed to determine whether TBMS1 induced cell cycle arrest or not. When T24 cells were treated with TBMS1, it caused the increased accumulation of ells in the G2/M phase. The percentage of G2/M phase were increased from 3.8 ± 1.4% in untreated cells to 14.6 ± 3.5%, and 38.1 ± 2.9% in the cells after treatment with 10 and 20 µM of TBMS1, respectively for 24 hours. This increase was coupled with the decreased percentage of cells in G0/G1 phase (Figure 3). These findings revealed that G2/M phase cell cycle arrest was one of the mechanisms through which TBMS1 induces cytotoxicity in T24 cells. A number of recent studies have shown that by arresting the cell cycle, several apoptosis is, therefore, most important in the treatment of cancer (Khan et al., 2011; Khan et al., 2012).
Figure 3: Effect of TBMS1 on cell cycle distribution. T24 cells were treated with 0, 10, and 20 µM of TBMS1 for 24 hours and then stained with PI for flow cytometric analysis. Histograms show number of cells/channel (y-axis) vs. DNA contents (x-axis). The values indicate the percentages of cells in the indicated phases of cell cycle. The data shown are representative of three independent experiments with the similar results. ap<0.05 and bp<0.01 compared with the control
Apoptosis, autophagy, and necrosis are the main modes of cell death (Leist and Jaattela, 2001). Among these pathways of cell death, apoptosis is most well planned and orderly mode of cell death (Elmore, 2007; Hengartner, 2000). More than 50% of neoplasm's undergo aberrations in the apoptotic machinery which leads to abnormal cell proliferation (Mashima and Tsuruo, 2005; Pommier et al., 2004). The regulation of apoptosis is, therefore, the most important in the treatment of cancer (Fulda, 2010; Lawen, 2003; Reed, 2002). Accumulated evidences indicated that the most of chemotherapeutic agents halt tumor cells proliferation via induction of apoptosis (Rasul et al., 2013a; Rasul et al., 2013b). TBMS1-induced cell death could be associated with apoptosis and necrosis (Kanduc et al., 2002; Kerr et al., 1972). To determine whether TBMS1 induces apoptosis or necrosis, we analyzed the rate of apoptosis induced by TBMS1 in T24 cells by double staining FITC annexin-V and PI. It was observed that the apoptosis rates were 17.05 and 29.62% in the cells treated with 10 and 20 µM TBMS1 for 24 hours respectively compared to the control cells at 4.04% (Figure 4). TBMS1-induced apoptosis in T24 cells was consistent with previously reported studies in lung, cervical, and leukemia cancer cells (Weng et al., 2003; Xu et al., 2009; Zhang et al., 2011)
Figure 4: Apoptosis induced by TBMS1 in T24 cells. T24 cells were treated with 0, 10, and 20 µM of TBMS1 for 24 hours. Then cells were stained with FITC-conjugated Annexin V and PI for flow cytometric analysis. The flow cytometry profile represents Annexin V-FITC staining in x axis and PI in y axis. The number represents the percentages of apoptotic cells in each condition. The data shown are representative of three independent experiments with the similar results. *p<0.05 and **p<0.01 compared with the control
Many studies revealed that mitochondrion is one of the essential components of the apoptosis execution machinery, which contain pro-apoptotic proteins (e.g., cytochrome c) (Elmore, 2007). It has been elucidated that upon the depolarization of the mitochondrial membrane potential results in the mitochondrial swelling and subsequent release of cytochrome c from the intermitochondrial membrane spaces into the cytosol (Buytaert et al., 2007). It is becoming increasingly apparent that mitochondria play a fundamental role in the processes those lead to the cell death (Wang, 2001). To probe the effect of TBMS1 on the ΔΨm, T24 cells were stained with the Rho-123 to measure the mitochondrial transmembrane potential. Effects of TBMS1 on the mitochondrial membrane potential of T24 cells were examined by flow cytometry using rhodamine-123 staining. The loss of mitochondrial membrane potential was considered directly proportional to the decrease of rhodamine-123 fluorescence in treated cells as compared to the normal cells. The rates of depletion of mitochondrial membrane potential were 84.3 ± 1.94% and 72.9 ± 1.6% in the cells treated with 10 and 20 µM of TBMS1 for 24 hours respectively as compared to 96.3 ± 0.8% in control group (Figure 5). The results indicated the depletion of mitochondrial transmembrane potential by TBMS1 in a time-dependent manner in T24 cells. Our data corroborate with the previously reported results that flavonoid induced dissipation of mitochondrial membrane potential, which provide the evidence for direct contribution of mitochondria in the natural compound-induced apoptosis (Khan et al., 2012).
Figure 5: The effects of TBMS1 on mitochondrial transmembrane potential of T24 cells were determined by flow cytometry. The values indicate the percentages of rhodamine 123 fluorescence in the T24 cells treated with 0, 10, and 20 µM of TBMS1 or without (control) for 24 hours The data shown are representative of three independent experiments with the similar results. ap<0.05 and bp<0.01 compared with the control
Interplay between proapoptotic (Bax) and antiapoptotic (Bcl-2) members of the Bcl-2 family pedals the mitochondrial apoptotic pathway (Mallat and Tedgui, 2000). Bcl-2 family proteins are pivotal for rising perme ability of mitochondrial membranes and the release of cytochrome c, which activates caspases and in turn mobilizes apoptotic cell death (Adams and Cory, 2007; Burlacu, 2003; Danial, 2007). The release of cytochrome c from the mitochondria into cytosol is coupled with the ratio of Bax/Bcl-2 proteins (Kluck et al., 1997). Bcl-2 family proteins are mostly involved in the mitochondrial apoptotic pathway (Reed, 1998). Furthermore, Bcl-2 family plays a central role in activation of caspases (Burlacu, 2003). Bcl-2 and Bax work in an antagonistic manner, Bcl-2 is antiapoptotic while Bax is proapoptotic member of Bcl-2 family and Bcl-2 forms heterodimers with Bax. Bax inserts into outer membrane of mitochondria under stress conditions, as a result transmembrane permeability become higher which facilitate the release of cytochrome C due to formation of pores on the mitochondrial outer membrane (Adams and Cory, 2007; Danial, 2007). The balance between these two groups is critical in the cell decision to undergo apoptosis or not (Mallat and Tedgui, 2000). Therefore, we performed western blotting to examine the effects of TBMS1 on the expression of Bcl-2 and Bax in T24 cells. TBMS1 was observed to be involved in the up-regulation of Bax and down regulation of Bcl-2 in a time-dependent manner (Figure 6). This results in a linear association between the mitochondria and TBMS1-induced apoptosis. These results are compatible to the previous report on TBMS1-induced apoptosis (Xu et al., 2009).
Figure 6: T24 cells were exposed to 0, 10, and 20 µM of TBMS1 for specified time intervals. Equal amounts of lysate protein were subjected to gel electrophoresis. Expression levels of Bcl-2 and Bax were monitored by Western blot assay. beta-actin was used as loading control. Data are representative of at least two independent experiments with similar results
Because p21WAF1/Cip1 is a cyclin-dependent kinase inhibitor and involved in cell growth arrest (Boulaire et al., 2000), we further examined the expression of p21WAF1/Cip1 in the TBMS1 (10 and 5 µM) treated T24 cells. Figure 7 showed that treatment of T24 cells with zinc (10 and 5 µM) resulted in the increased expression of p21WAF1/Cip1, suggesting that TBMS1 induces apoptosis in the bladder cancer cells via up-regulation of p21WAF1/Cip1 expression.
Figure 7: The p21Waf1/Cip1 up-regulation and activation corresponding to apoptotic process induced by TBMS1. Tubeimoside-1 augmented p21WAF1/Cip1 expression. T24 cells were exposed to 5, 10 and 20 µM TBMS-1 for 24 hours. The p21WAF1/Cip1 levels were examined by immunoblot analysis with either p21WAF1/Cip1 or beta-actin antibody. T24 cells were cotransfected with 25 ng of Renilla luciferase reporter and 100 ng of luciferase reporter, p21P-luc as indicated, and then were exposed to various concentration of TBMS for 8 hours. Relative luciferase activities were measured
Previous studies have shown that p21WAF1/Cip1 is a potent cell cycle inhibitor downstream of p53 (Boulaire et al., 2000; Chi et al., 2005; Derynck et al., 2001; Feng et al., 2002; Pardali et al., 2005; Saramaki et al., 2006) To determine how zinc induces p21WAF1/Cip1 transactivation, p21WAF1/Cip1 promoter-driven luciferase comparative studies were initially conducted. As shown in Figure 2B, there were significant elevation of p21WAF1/Cip1 promoter-driven luciferase activities for p21P-luc reporters in the TBMS1 treated T24 cells in a dose-dependent manner, reaching maximal level which is about 3-fold of control after 20 µM tbms1 concentration treatment. These results showed that the p21WAF1/Cip1 promoter was capable of being activated by TBMS1.
Conclusion
TBMS1 inhibits cell proliferation of human bladder cancer T24 cells. Further characterization shows that TBMS1 effectively inhibited the proliferation of T24 cells through arresting cell cycle at G2/M phase and induction of apoptosis which is regulated by down-regulation of Bcl-2 and up-regulation of Bax and p21. These in vitro results suggest that TBMS1 should be further examined for its therapeutic value in human bladder cancer.
References
Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26: 1324-37.
Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009; 27: 2712-25.
Boulaire J, Fotedar A, Fotedar R. The functions of the cdk-cyclin kinase inhibitor p21WAF1. Pathol Biol (Paris). 2000; 48: 190-202.
Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003; 7: 249-57.
Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007; 1776: 86-107.
Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, Kim HR, Cha EJ, Lee YH, Kaneda A, Ushijima T, Kim WJ, Ito Y, Bae SC. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation with transforming growth factor {beta}-activated SMAD. Mol Cell Biol. 2005; 25: 8097-107.
Danial NN. BCL-2 family proteins: Critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007; 13: 7254-63.
Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001; 29: 117-29.
Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007; 35: 495-516.
Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate 2002; 52: 311-18.
Fulda S. Evasion of apoptosis as a cellular stress response in cancer. Int J Cell Biol. 2010; 2010: 370835.
Grana X, Reddy EP. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995; 11: 211-19.
Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770-76.
Kanduc D, Mittelman A, Serpico R, Sinigaglia E, Sinha AA, Natale C, Santacroce R, Di Corcia MG, Lucchese A, Dini L, Pani P, Santacroce S, Simone S, Bucci R, Farber E. Cell death: Apoptosis versus necrosis (review). Int J Oncol. 2002; 21: 165-70.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26: 239-57.
Khan M, Rasul A, Yi F, Zhong L, Ma T. Jaceosidin induces p53-dependent G2/M phase arrest in U87 glioblastoma cells. Asian Pac J Cancer Prev. 2011; 12: 3235-38.
Khan M, Yu B, Rasul A, Al Shawi A, Yi F, Yang H, Ma T. Jaceosidin Induces Apoptosis in U87 Glioblastoma Cells through G2/M Phase Arrest. Evidence-based complementary and alternative medicine: eCAM 2012; 2012: 703034.
Kintzios SE, Barberaki MG. Plants that fight cancer. CRC Press: Boca Raton, 2004.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997; 275: 1132-36.
Lawen A. Apoptosis: An introduction. BioEssays 2003; 25: 888-96.
Lee SH, Lee MY, Kang HM, Han DC, Son KH, Yang DC, Sung ND, Lee CW, Kim HM, Kwon BM. Anti-tumor activity of the farnesyl-protein transferase inhibitors arteminolides, isolated from Artemisa. Bioorg Med Chem. 2003; 11: 4545-49.
Leist M, Jaattela M. Four deaths and a funeral: From caspases to alternative mechanisms. Nature Rev Mol Cell Biol. 2001; 2: 589-98.
Ma R, Song G, You W, Yu L, Su W, Liao M, Zhang Y, Huang L, Zhang X, Yu T. Antimicrotubule activity of tubeimoside I and its colchicine binding site of tubulin. Cancer Chemother Pharmacol. 2008; 62: 559-68.
Magadula JJ, Erasto P. Bioactive natural products derived from the East African flora. Nat Prod Reports. 2009; 26: 1535-54.
Mallat Z, Tedgui A. Apoptosis in the vasculature: Mechanisms and functional importance. Br J Pharmacol. 2000; 130: 947-62.
Mashima T, Tsuruo T. Defects of the apoptotic pathway as therapeutic target against cancer. Drug Resist Updat. 2005; 8: 339-43.
Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1). J Cell Physiol. 2005; 204: 260-72.
Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009; 27: 289-93.
Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene 2004; 23: 2934-49.
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M, Ma T. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis 2012a; 17: 1104-19.
Rasul A, Song R, Wei W, Nishino Y, Tsuji I, Li X, Li J. Tubeimoside-1 inhibits growth via the induction of cell cycle arrest and apoptosis in human melanoma A375 cells. Bangladesh J Pharmacol. 2012b; 7: 150-56.
Rasul A, Bao R, Malhi M, Zhao B, Tsuji I, Li J, Li X. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules 2013a; 18: 1418-33.
Rasul A, Di J, Millimouno FM, Malhi M, Tsuji I, Ali M, Li J, Li X. Reactive oxygen species mediate isoalantolactone-induced apoptosis in human prostate cancer cells. Molecules 2013b; 18: 9382-96.
Reed JC. Bcl-2 family proteins. Oncogene 1998; 17: 3225-36.
Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002; 1: 111-21.
Saramaki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006; 34: 543-54.
Tan RX, Zheng WF, Tang HQ. Biologically active substances from the genus Artemisia. Planta Med. 1998; 64: 295-302.
Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003; 36: 131-49.
Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001; 15: 2922-33.
Weng XY, Ma RD, Yu LJ. [Apoptosis of human nasopharyngeal carcinoma CNE-2Z cells induced by tubeimoside I]. Chinese J Cancer. 2003; 22: 806-11.
Woldemichael GM, Wink M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J Agric Food Chem. 2001; 49: 2327-32.
Xu Y, Chiu JF, He QY, Chen F. Tubeimoside-1 exerts cytotoxicity in HeLa cells through mitochondrial dysfunction and endoplasmic reticulum stress pathways. J Proteome Res. 2009; 8: 1585-93.
Yu L, Ma R, Yu T. Induction of morphological and functional differentiation of human promyelocytic leukemia cells (HL-60) by tubeimoside 1. Planta Medica. 1996; 62: 119-21.
Yu LJ, Ma RD, Wang YQ, Nishino H, Takayasu J, He WZ, Chang M, Zhen J, Liu WS, Fan SX. Potent anti-tumorigenic effect of tubeimoside 1 isolated from the bulb of Bolbostemma paniculatum (Maxim) Franquet. Int J Cancer J Int du Cancer. 1992; 50: 635-38.
Yu LJ, Ma RD, Jiang SB. Effects of tubeimoside-1 on HIV core protein p24 and cytopathogenesis in vitro. Zhongguo Yao Li Xue Bao (Acta Pharmacologica Sinica). 1994; 15: 103-06.
Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep. 2011; 4: 25-29.