Phytochemical screening and antimicrobial activity of extracts from leaves and stem of Ecbolium linneanum
Abstract
The soxhlet extracts of leaves and stem of Ecbolium linneanum using different solvents were investigated for their phytochemical and antibacterial activity against pathogenic bacteria. Antibacterial activity was performed by using well agar and disc diffusion methods. Preliminary phytochemical screening revealed that leaves and stem contained various phytochemicals and were confirmed by spectroscopic methods. The bacterial pathogens were strongly inhibited by leaf extracts but acetone extracts of stem have failed to inhibit the growth of Staphylococcus aureus and Pseudomonas aeruginosa even at the highest concentration. The results revealed that leaf extracts were found to be more effective than stem extracts. E. linneanum possesses antimicrobial activity against most commonly encountered human pathogens.
Introduction
Plants are used as food, flavor, cosmetic, ornamental, fumigant, insect deterrent, and medicine (Nitto et al., 2002). Over centuries and decades our ancestors relied on the herbal products as therapeutic which can be traced back to at least 5,000 years (Rekha and Sundararajan, 2010). According to World Health Organization about 80% of the world population depends on the natural product for their health due to minimal side effect and cost effective (Jagtap et al., 2009). In recent years, there has been a global increase to screen the biological activities on plants. Plants are well known to produce certain bioactive molecules which react with other organisms in the environment (Harborne and Baxter, 1995). Infectious diseases caused by microorganisms have developed resistance to many antibiotics and this has created immense clinical problem in the treatment of infectious diseases. Therefore, scientists are forced to search for new antimicrobial substances from various sources including medicinal plants because of the less availability and high cost of new generation antibiotics (Sashikumar et al., 2003).
Ecbolium linneanum belongs to the family Acanthaceae commonly referred to as Blue Fox Tail or Blue Justicia in English, Neel Kantha in Bengali, Udajatiin Hindi and Nilambari in Tamil. It is an indigenous plant grows naturally along the Eastern part of India and also found in Africa and tropical Asia. There were claims from the local native practitioner that the decoction of E. linneanum is highly useful in treating diseases like jaundice, menorrhoea, rheumatism (Chopra et al., 1956) and anti-inflammatory activity (Lalitha and Sethuraman, 2010). Root juice is used as antihelmintic and also to treat premenstrual colic (Sharma and Sharma, 2010). There is no reports on the antimicrobial activity of leaf and stem extract of E. linneanum. Therefore, the present study aimed to screen the phytochemicals and to evaluate the antibacterial activity against human pathogens.
Materials and Methods
Chemicals
All solvents ethanol, acetone, dichloromethane and petroleum ether were purchased from Merck, India. Nutrient broth for bacterial culture and Mueller-Hinton agar medium for antimicrobial activity were purchased from Hi-Media, India. All chemicals used in the study were of analytical grade.
Collection and processing of plant samples
Healthy, disease free leaf and stem of E. linneanum were collected during the month of May, 2010 in and around the villages of Bankura district of West Bengal, India. The collected plants with complete herbarium was identified and authenticated from the Department of Botany, PSGR Krishnammal College for Women, Coimbatore, South India. The collected leaf and stem were washed properly in the tap water followed by detergent and finally rinsed with distilled water until no foreign material remained (damaged leaves were removed). The fresh plant materials were left to dry in a closed room (25-28ºC) for approximately 10 days. The dried plant parts were pulverized, using sterile electrical blender to obtain powder. The powdered samples were stored in air tight container, protected from sunlight for further use.
Extract preparation
Twenty five grams of powdered plant materials were continuously extracted with different solvents like ethanol, acetone (80%), dichloromethane and petroleum ether for successive solvent extraction based on polarity using soxhlet extraction apparatus at the boiling point of the respective solvents for 12-16 hours or until the color of the extracted solvent became clear. Different extracts were concentrated under reduced pressure using rotary evaporator and they were poured into a weighed vial, further dried in a desiccating chamber until a constant dry weight was obtained. The residues were stored at 4ºC for further studies. The yield from 100 g of dried powdered material was calculated as follows:
Amount of product
Product yield = ------------------------------------------- X 100
Amount of sample added
Phytochemical analysis
Phytochemical analysis of the leaf and stem extracts of E. linneanum were analyzed using standard qualitative methods as described by Harborne and Baxter (1995). The compounds analysed for phytochemicals were alkaloids, carbohydrates, flavonoids, glycosides, phenols, phytosterols, proteins, resins, saponins, tannins and thiols.
Gas chromatography mass spectroscopy (GCMS) analysis
Among all other extracts only the ethanolic extract of leaves was selected to identify the chemicals using GCMS at South Indian Textile Research Association (SITRA), Coimbatore, South India by Thermo GC - Trace Ultra Version: 5.0, Thermo MS DSQ II equipment in TR5 - Ms capillary standard non-polar column (dimension: 30 m, ID: 0.25 mm, film: 0.25 µm). The chromatogram obtained from the GC was then analyzed in the mass spectrography (MS) to get the mass of all the fractions. The identification of compounds was accomplished using computer searches in plant compound library.
Fourier transform infrared spectroscopy (FTIR) analysis
The FTIR analysis of aqueous leaf extract of E. linneanum using FTIR Shimadzu--8400S was carried out at PSG College of Arts and Science, Coimbatore, South India using KBR pellet. The FTIR was recorded in the range of 400 to 4,000 cm-1. The various modes of vibrations were identified and assigned to know the different functional groups present in the extract.
Bacterial pathogens and their growth conditions
The pathogens Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa were obtained from the Microbiology Laboratory, Kovai Medical Centre and Hospital Coimbatore, India. The strains were maintained on nutrient agar slants at 4ºC.
Preparation of inoculums
Stock cultures were maintained at 4°C on slopes of nutrient agar. Active cultures for experiments were prepared by transferring a loopful of cells from the stock cultures to test tubes of Mueller-Hinton broth (MHB) that were incubated without agitation for 24 hours at 37°C. The cultures were diluted with fresh MHB to achieve optical densities corresponding to 0.5 i.e. 105 to 106 CFU/mL using McFarland's standard.
Agar well diffusion method
Mueller-Hinton agar plates were swabbed on three axes with sterile cotton tipped swab, which were dipped in the freshly prepared diluted culture. A 6 mm hole was bored aseptically using sterile cork borer. The agar plugs were taken out carefully so as not to disturb the surrounding medium. The holes were filled with different concentrations of leaf and stem extracts (50, 100, 150, 200 and 250 mg/mL) and allowed to stand for one hour for perfusion of the extracts (Esimone et al., 1998) and kept for incubation at 30ºC for 24 hours. After incubation, the petri plates were observed for the antibacterial activity and measured in terms of diameter in millimetre of the inhibition zone. The respective solvents were used as negative control and the antibiotics like kanamycin (30 ug), norfloxacin (10 ug) and ciprofloxacin (5 ug) were used as positive control.
Statistical analysis
The antimicrobial activity was determined by measuring the diameter of zone of inhibition that is the mean of triplicates ± SD.
Results
The obtained yield of the plant extracts differed between the leaf and stem as well as with the different solvents used for extraction (Table I). Among the extracts, ethanol extracted highest yield (5.5 g/100 g) from E. linneanum leaf whereas petroleum ether extract of stem yield was found to be low (0.7 g/100 g).
Table I: Yield of plant extracts of E. linneanum in different solvents
| Solvents used | %Yield from plant powder (g) | |
|---|---|---|
| Leaf | Stem | |
| Ethanol | 5.5 | 2 |
| Acetone (80%) | 4 | 3.5 |
| Dichloromethane | 2.9 | 0.9 |
| Petroleum ether | 1.4 | 0.7 |
Preliminary phytochemical screening revealed the presence of alkaloids, carbohydrates, flavonoids, glycolsides, phenols, phytosterols, proteins, resins, saponins, tannins and thiols (Table II). All the phytochemicals were present in aqueous extracts of leaf but in the case of aqueous extract of stem, phenols, phytosterols and resins were absent. In the acetone extract of stem of E. linneanum, all the phytochemicals were present but in the case of ethanolic extract of stem, flavonoids, glycosides and saponins were absent. However in E. linneanum leaf, all the phytochemicals were present in ethanolic and acetone extracts but in the case of petroleum ether extract of leaf only flavonoids and glycosides are present.
Table II: Phytochemicals of leaves and stem extracts of E. linneanum
| Compounds | Leaves extracts | Stem extracts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Acetone | DCM | Ether | Aqueous | Ethanol | Acetone | DCM | Ether | Aqueous | |
| Alkaloid | + | + | + | - | + | + | + | + | - | + |
| Carbohydrate | + | + | - | - | + | + | + | - | - | + |
| Flavonoid | + | + | + | + | + | + | + | + | + | |
| Glycoside | + | + | + | + | + | + | + | + | + | |
| Phenol | + | + | - | - | + | + | + | - | - | - |
| Phytosterol | + | + | + | - | + | + | + | - | + | - |
| Protein | + | + | - | - | + | + | + | + | - | + |
| Resin | + | + | - | - | + | + | + | - | - | - |
| Saponin | + | + | - | - | + | - | + | - | - | + |
| Tannin | + | + | - | - | + | + | + | - | - | + |
| Thiol | + | + | + | - | + | + | + | + | - | + |
| "+" Represents presence of the phytoconstituent; "-” represents absence of the phytoconstituent | ||||||||||
Ethanolic extracts of leaf was subjected to GCMS analysis because the preliminary phytochemical screening showed the presence of phytochemicals (Figure 1). The probable compounds present (Table III) in the leaf extracts were searched in the plant compound library with respect to the mass and retention time of each fraction from the column.
Figure 1: GC-MS chromatogram of ethanolic leaf extract of E. linneanum
Table III: List of phytochemical constituents identified by GC-MS spectra
| Probable compound name | Molecular formula | M.W. | Retention time (min) | Area (%) | Probable compound structure |
|---|---|---|---|---|---|
| (R)-4-( 1',1'-Dimethylethyl)-1,3,2-dioxathiolane-2-one | C6H12O3S | 164 | 15.7 | 8.4 | 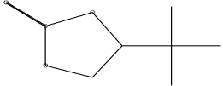 |
| Neophytadiene | C20H38 | 278 | 20.2 | 6.1 | |
| 3,5-Dioxohexanoic acid | C6H8O4 | 144 | 23.2 | 7.6 | 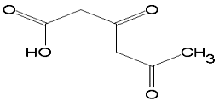 |
| 3-Chloromethylfuran | C5H5ClO | 116 | 25.3 | 6.2 |  |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- (CAS) | C19H32O2 | 292 | 26.3 | 10.1 | 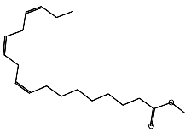 |
| 1-(Cyclohexan-1-yl)but-3-ene | C10H16 | 136 | 33.9 | 7.5 | 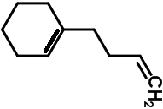 |
The functional groups of the compounds present in the aqueous leaf extract were identified by the FTIR spectra. Major peaks in the FTIR spectrum of E. linneanum leaf aqueous extract (Figure 2) are ∼601, ∼1049, ∼1388, ∼1620, ∼2924, ∼3356 cm-1 and the minor picks are ∼3796, ∼2283, ∼1226, ∼902 cm-1. The FTIR spectrum exhibited the characteristics finger print band features. The very strong absorption at 3356 cm-1 represents O-H stretching vibration and also characteristic of the presence of N-H primary amines (Silverstein et al., 1981). The spectrum exhibits intense bands at ∼2924 assigned to the symmetric stretching vibration of sp3 hybridized -CH2 groups (Mukherjee et al., 2008) and the wide absorption spectra at about 1620 cm-1 may result from stretching of vibration of -C=C- (Huang et al., 2007). The 1388 cm-1 is showing CH3 deformation can be assigned to germinal methyls (Shankar et al., 2004) and at ∼1226 cm-1 band arises most probably from the C-O group of polyols such as hydroxyflavones and catechins (Jain et al., 2009). The absorption peak at around 1049 cm-1 can be assigned as absorption peaks of the ether linkages or -C-O-C- or -C-O-. To a large extent, the band at ∼1049 cm-1 might be contributed by the -C-O- groups of the polyols such as flavones, terpenoids and the polysaccharides present in the biomass (Huang et al., 2007). The absorbance peak between 941-803 cm-1 indicate C-H plane deformation.
Figure 2: FTIR chromatogram of ethanolic leaf extract of E. linneanum
Antibacterial effect of the leaf and stem extracts of E. linneanum was quantitatively assessed on the basis of zone of inhibition. According to Clinical and Laboratory Standard Institutes (2006) guidelines, the test bacterial pathogens were resistant to all three antibiotics (positive control). Different extracts of leaf and stem of E. linneanum, have exhibited different degrees of antibacterial activity against bacterial pathogens (Table IV). Similarly, the inhibition zones formed by standard antibiotics are shown in Table V. When the extracts of leaf tested against the pathogenic bacteria and found that their growth were strongly inhibited at different concentrations. Moreover S. aureus and E. faecalis were inhibited even at the lowest concentration (50 mg/mL) except petroleum ether extract, where their growth was inhibited only at 100 mg/mL. Similarly, when the extracts of the stem tested against the pathogenic bacteria, it was observed that the growth of the pathogenic bacteria was inhibited by ethanolic extract even at lowest concentration. Whereas the other extracts were able to inhibit the growth of pathogenic bacteria at varying concentration. But in the case of K. pneumoniae, the growths were not inhibited even at the highest (250 mg/mL) concentration of dichloromethane and petroleum ether extracts. When the ethanolic extracts of leaf and stem was compared, it was found that leaf extracts exhibited stronger inhibitory effect against S. aureus, P. aeruginosa and E. coli, whereas stem ethanol extract acted only against E. faecalis and K. pneumoniae with a zone of inhibition of 24.5 ± 0.4 mm and 18.4 ± 0.2 mm respectively. Among the extracts, leaf ethanol and dichloromethane extracts and stem ethanol extract exhibited greater inhibitory effect at 250 mg/mL than standard antibiotics. Thus the extracts of leaf and stem showed a varying degree of effectiveness against the pathogenic microorganisms.
Table IV: Antimicrobial activity of E. linneanum leaf and stem extracts
| Extracts | Zone of inhibition (mm) |
|||||
|---|---|---|---|---|---|---|
| Concentration (mg/mL) |
S. aureus | P. aeruginosa | E. coli | E. faecalis | K. pneumoniae | |
| Leaves ethanol extract | 50 | 8.1 ± 0.6 | 10.8 ± 0.3 | 9.5 ± 0.5 | 12.6 ± 0.5 | 7.3 ± 0.4 |
| 100 | 10.2 ± 0.3 | 13.6 ± 0.6 | 11.3 ± 0.4 | 14.5 ± 0.3 | 10.5 ± 0.3 | |
| 150 | 13.8 ± 0.6 | 15.3 ± 0.3 | 13.4 ± 0.6 | 17.5 ± 0.2 | 12.8 ± 0.2 | |
| 200 | 16.5 ± 0.4 | 16.3 ± 0.4 | 16.2 ± 0.4 | 19.4 ± 0.6 | 15.3 ± 0.4 | |
| 250 | 19.2 ± 0.2 | 20.8 ± 0.3 | 19.2 ± 0.6 | 21.3 ± 0.3 | 17.3 ± 0.3 | |
| Leaves acetone extract | 50 | 8.2 ± 0.3 | - | - | 8.5 ± 0.2 | - |
| 100 | 11.4 ± 0.2 | - | - | 10.2 ± 0.4 | - | |
| 150 | 14.2 ± 0.3 | - | 7.4 ± 0.2 | 13.1 ± 0.3 | 7.3 ± 0.2 | |
| 200 | 17.5 ± 0.6 | 9.3 ± 0.4 | 10.1 ± 0.2 | 17.3 ± 0.2 | 8.3 ± 0.2 | |
| 250 | 20.2 ± 0.4 | 12.9 ± 0.3 | 13.3 ± 0.6 | 22.2 ± 0.4 | 11.2 ± 0.3 | |
| Leaves dichloromethane extract | 50 | 7.3 ± 0.2 | 10.3 ± 0.5 | 10.2 ± 0.3 | 9.7 ± 0.2 | 6.5 ± 0.3 |
| 100 | 9.3 ± 0.1 | 12.6 ± 0.1 | 12.5 ± 0.4 | 11.2 ± 0.7 | 8.4 ± 0.2 | |
| 150 | 11.3 ± 0.7 | 15.3 ± 0.2 | 15.3 ± 0.2 | 13.4 ± 0.9 | 10.8 ± 0.2 | |
| 200 | 14.4 ± 0.3 | 18.5 ± 0.9 | 17.3 ± 0.7 | 16.5 ± 0.3 | 12.6 ± 0.8 | |
| 250 | 16.7 ± 0.4 | 21.6 ± 0.6 | 19.7 ± 0.3 | 18.3 ± 0.4 | 15.2 ± 0.3 | |
| Leaves petroleum ether extract | 50 | - | 6.2 ± 0.5 | - | - | - |
| 100 | 6.2 ± 0.3 | 7.3 ± 0.5 | 6.3 ± 0.6 | 6.3 ± 0.6 | - | |
| 150 | 7.3 ± 0.7 | 9.5 ± 0.4 | 8.4 ± 0.3 | 8.2 ± 0.4 | 6.3 ± 0.6 | |
| 200 | 10.4 ± 0.3 | 13.5 ± 0.6 | 10.3 ± 0.4 | 9.2 ± 0.4 | 8.3 ± 0.6 | |
| 250 | 12.7 ± 0.3 | 15.3 ± 0.4 | 13.3 ± 0.2 | 10.6 ± 0.2 | 10.4 ± 0.2 | |
| Stem ethanol extract | 50 | 7.2 ± 0.6 | 10.4 ± 0.7 | 7.7 ± 0.6 | 10.3 ± 0.4 | 7.4 ± 0.1 |
| 100 | 9.1 ± 0.4 | 12.7 ± 0.2 | 9.2 ± 0.6 | 12.5 ± 0.5 | 10.2 ± 0.3 | |
| 150 | 11.9 ± 0.2 | 15.6 ± 0.4 | 10.3 ± 0.7 | 15.4 ± 0.6 | 13.3 ± 0.7 | |
| 200 | 15.8 ± 0.4 | 17.1 ± 0.5 | 14.9 ± 0.3 | 19.0 ± 0.4 | 16.4 ± 0.7 | |
| 250 | 18.1 ± 0.2 | 19.3 ± 0.5 | 17.2 ± 0.5 | 24.5 ± 0.4 | 18.4 ± 0.2 | |
| Stem acetone extract | 50 | - | - | - | - | - |
| 100 | - | - | - | 6.5 ± 0.6 | - | |
| 150 | - | - | 7.2 ± 0.4 | 8.7 ± 0.5 | - | |
| 200 | - | - | 10.5 ± 0.4 | 11.9 ± 0.5 | 6.5 ± 0.1 | |
| 250 | - | - | 12.3 ± 0.6 | 14.1 ± 0.5 | 8.2 ± 0.6 | |
| Stem dichloromethane extract | 50 | - | 6.5 ± 0.3 | - | 6.6 ± 0.3 | - |
| 100 | - | 7.6 ± 0.3 | - | 8.2 ± 0.5 | - | |
| 150 | 7.0 ± 0.4 | 9.1 ± 0.4 | 6.8 ± 0.5 | 9.9 ± 0.2 | - | |
| 200 | 8.0 ± 0.5 | 10.7 ± 0.4 | 7.3 ± 0.4 | 11.5 ± 0.6 | - | |
| 250 | 10.0 ± 0.4 | 12.2 ± 0.9 | 9.2 ± 0.2 | 13.3 ± 0.4 | - | |
| Stem petroleum ether extract | 50 | - | - | - | - | - |
| 100 | - | - | - | - | - | |
| 150 | - | 6.6 ± 0.3 | - | 6.7 ± 0.5 | - | |
| 200 | - | 7.4 ± 0.1 | - | 7.2 ± 0.2 | - | |
| 250 | 8.1 ± 0.5 | 8.3 ± 0.2 | 7.1 ± 0.5 | 9.3 ± 0.3 | - | |
| " - " represents absence of zone of inhibition; Values are mean ± SD of three determinations | ||||||
Table V: Antimicrobial activity of standard antibiotics
| Microorganisms | Zone of inhibition (mm) |
||
|---|---|---|---|
| Kanamycin (30 ug) | Norfloxacin (10 ug) | Ciprofloxacin (5 ug) | |
| S. aureus | 8 | 10 | 12 |
| P. aeruginosa | 10 | 10 | 14 |
| E. coli | 10 | 14 | 18 |
| E. faecalis | 11 | 10 | 14 |
| K. pneumonia | 12 | 16 | 18 |
Discussion
Among the solvents, extract yield was higher in leaf and stem when ethanol was used as the extracting solvent, whereas acetone extracted higher yield only in stem. On the other hand, there was a decrease in yield with the polarity of the solvent used for extraction. The differences in the extract yields from the tested plant materials in the present analysis might be ascribed to the different availability of extractable components, resulting from the varied chemical composition of plants (Hsu et al., 2006). The amount of antioxidant components that can be extracted from a plant material is mainly affected by the extracting solvent, which may probably vary from sample to sample. Phytochemical screening of the plant revealed some differences in the constituents of the extracts tested. E. linneanum aqueous extract tested positive for all the phytochemical constituents. It is clear that the extraction of phytochemicals gradually reduced with the polarity of solvents used for extraction. The preliminary phytochemical analysis were established by GC-MS and FTIR of the ethanolic and aqueous extracts of leaf of the plant respectively. From GC-MS spectra, some of the major compounds identified were mostly of phenols, alkaloids, terpenoids, amines, acids and ethers etc. The major compounds confirmed by FTIR analysis are phenols, polyols, amines, aldehyde, ketones, ethers, alkaloids, flavones, terpenoids, proteins and the polysaccharides etc. So the primary phytochemical analysed by different biochemical colour formation reactions were confirmed with the help of GC-MS and FTIR spectroscopic methods.
Both leaf and stem of the plant used in the study have been used traditionally for the treatment of various diseases including those caused by the microorganisms. Ethanolic and acetone extracts of leaf as well as ethanolic extract of stem were found to be highly effective against the bacterial pathogens and also possessed greater inhibitory effect than antibiotics. Some of the phytochemical compounds like glycosides, saponins, tannins, flavonoids, terpenoids, alkaloids, have variously been reported to have antimicrobial activity (Okeke et al., 2009). E. linneanum also possessed alkaloid substances which also have biological activities. In this study, some of the bacterial pathogens were not inhibited, whereas some extracts showed broad-spectrum activity. This might be due to masking of antibacterial activity by the presence of some inhibitory compounds or factors in the extract which varied by the polarity of the solvents used for extraction. Higher zone of inhibition against the bacterial pathogens exhibited by the extracts is of great significance in the health care delivery system, since it could be used as an alternative to orthodox antibiotics in the treatment of infections, especially as they frequently develop resistance to known antibiotics (Singleton, 1999). Considering the rich diversity of plants, it is expected to screen and scientifically evaluate the plant extracts for their phytochemicals and antimicrobial activity.
Conclusion
In a nutshell, the current study on leaf and stem extracts of E. linneanum showed their potential antimicrobial activities against the bacterial pathogens and provide an ample opportunity to plant based drug designing due to considerable role of phytochemicals in the ethnomedicine.
Acknowledgements
The authors would like to thank Mr. Sukumar Choudhury (Barasat, Bankura, West Bengal, India) for his kind help in plant collection, Dr. Sasi (Department of Botany, PSGR Krishnammal College for Women, Coimbatore) for providing the identification of E. linneanum, Dr. S. Kavitha for proof reading. Authors are also grateful to Drs. Paul Dhinakaran (Chancellor), Paul P. Appasamy (Vice Chancellor) and Annie Mary Fernandez (Registrar) of Karunya University, Coimbatore, India for providing laboratory facilities and kind support to carry out this publication.
References
Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi, CSIR, 1956.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. CLSI approved standard. Clinical and Laboratory Standards Institute, Wayne PA, 2006, M-100-S16 (M7).
Esimone CO, Adiukwu MU, Okonta JM. Preliminary antimicrobial screening of the ethanolic extract from the Lichen Usnea subfloridans (L). J Pharma Res Dev. 1998; 3: 99- 102.
Harborne JB, Baxter H. Phytochemical dictionary: A handbook of bioactive compounds from plants. London, Taylor & Francis Ltd, 1995.
Hsu B, Coupar IM, Ng K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem. 2006; 98: 317-28.
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C. Biosynthesis of silver and gold nanoparticles by novel sun dried Cinnamomum camphora leaf. Nanotechnology 2007; 18: 105104.
Jagtap NS, Khadabadi SS, Ghorpade DS, Banarase NB, Naphade SS. Antimicrobial and antifungal activity of Centella asiatica (L.) Urban Umbeliferae. Res J Pharm Technol. 2009; 2: 328-30.
Jain D, Daima HK, Kachhwaha S, Kothari SL. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig J Nanomaterials Biostruct. 2009; 4: 557-63.
Lalitha KG, Sethuraman MG. Anti-inflammatory activity of roots of Ecbolium viride (Forsk) Merrill. J Ethnopharmacol. 2010; 128: 248-50.
Mukherjee P, Roy M, Mandal BP, Dey GK, Mukherjee PK, Ghatak J, Tyagi AK, Kale SP. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008; 19: 75-103.
Nitto T, Arai T, Takamatsu H, Inatomi Y, Murata H, Iinuma M, Tanaka T, Ito T, Asai F, Ibrahim I, Nakanishi T, Watabe K. Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J Health Sci. 2002; 48: 273-76.
Okeke MI, Iroegbu CU, Eze EN, Okoli AS, Esimone CO. Evaluation of extracts of the root of Landolphia owerrience for antibacterial activity. J Ethnopharmacol. 2001; 78: 119-27.
Rekha R, Sundararajan R. Preliminary phytochemical analysis and anti-bacterial activity of Mimosa pudica Linn leaves. Int J Pharma Bio Sci. 2010; 1: 1-8.
Sashikumar JM, Remya M, Janardhanan K. Antimicrobial activity of ethanol medicinal plants of Nilgiri Biosphere reserve and Western Ghats. Asian J Microbiol Biotechnol. 2003; 5: 183-85.
Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Aucore Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Coll Inter Sci. 2004; 275: 496-502.
Sharma R, Sharma HK. Ethnomedicine of Sonarpur, Kamrup district, Assam. Ind J Trad Know. 2010; 9: 163-65.
Silverstein RM, Bassler GC, Morrill TC. Spectrometric identification of organic compounds. 4th ed. New York, John Wiley and Sons, 1981.
Singleton P. Bacteria in biology, biotechnology and medicine. 4th ed. New York, John Wiley and Sons Ltd, 1999.

