Synthesis, structural characterization and biological activity of 2-(4-methylbenzenesulphonamido)pentanedioic acid amide derivatives: In vitro and in vivo antineoplastic activity
Abstract
In the present work few novel 2-(4-methylbenzenesulphonamido)pentanedioic acid amide derivatives and the basic compound 2-(4-methylphenylsulfonamido)pentanedioic acid have been synthesized, characterized and screened for their possible antineoplastic activity both in vitro and in vivo. The in vitro activity was performed against five human cell lines like human breast cancer (MCF-7), leukemia (K-562), ovarian cancer (OVACAR-3), human colon adenocarcinoma (HT-29) and Human kidney carcinoma (A-498). The in vivo activity was performed in female Swiss albino mice against Ehrlich ascites carcinoma. Among the synthesized compounds, ureide, p-bromoanilide, p-nitoanilide, o-bromoanilide and p-methylanilide derivatives of 2-(4-methyl benzene sulphonyl)-pentanedioic acid amides showed encouraging activity in both the in vitro and in vivo compared to other compounds. It was noticed that final derivative compounds show a better activity than the parent compound and it may be due to the substituents present in those compounds.
Introduction
Glutamic acid (2-amino pentanedioic acid) plays an important role in the biosynthesis of purine and pyrimidine bases of DNA and RNA (Rodwell, 2000). It is metabolized to L-glutamine by L-glutamine synthetase and this metabolic process is essential for normal maintenance of cells. The synthesis of L-glutamine is hindered in neoplastic cells due to lower reactivity of L-glutamine synthetase. Thus antagonists of this enzyme can interfere with the metabolic role of L-glutamine and act as anticancer agents (Hartman, 1970). Azaserine and 6-diaza-5-oxo-L-norleucine antagonized the metabolic process involving L-glutamine and exhibited antitumor activity in animal models (Eidinoff, 1958). L-glutamic acid gamma-(4-hydroxyanilide) a growth regulatory substance isolated from mushroom Agaricus bisporous was found to inhibit B16 mouse melanoma cells in culture. The importance of non-essential amino acid glutamine in proliferation of human tumor cells was studied extensively (Graff et al., 1940; Petit, 1977). All tumor cells studied were found to have a high activity of phosphate-dependent glutaminase utilizing glutamine from the medium during long-term culture (Graff et al., 1940). Human hepatoma cells take up glutamine at rates several fold faster than the normal human hepatocytes (Petit, 1977). L-Glutamine is not only the precursor of the biosynthesis of purine and pyrimidine bases of DNA as well as used as a building block of proteins. Other than glucose it is one of the major substrates for the energy metabolism of rapidly growing tumor cells. Beside glucose, glutamine is assumed to be the main energy source in tumor cells (Keren and Stark, 1988). Since cells, whether cancerous or normal, cannot survive without the only circulatory sugar glucose whereas glutamine is a nonessential amino acid which is required by most of the cells and tissues. It also considered being the most essential component of tissue/cell culture media for not only as the nitrogen source but also as the carbon source. Since most of the cells need glutamine for physiological functions and most of those normal cells are transformable to cancerous one, glutamine may play a significant role in cancer (Rosowsky et al., 1979; Debnath et al., 2002). Thus, the structural variants of glutamine attracted our attention to develop possible anticancer agents, which may act through glutamine and/or folic acid antagonism.
Materials and Methods
Commercially available reagents and starting materials for the synthesis were obtained from E. Merck, India, CDH, s.d. Fine Chem, India and Qualigens, India. Silica gel G used for TLC was obtained from E. Merck. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60F-2c54) using UV light as visualizing. Melting points were determined in an open glass capillary using a Kjeldahl flask containing paraffin and are uncorrected. The proton and carbon magnetic resonance spectra (1H NMR, 13C NMR) were recorded on a Bruker 400 MHz instrument (Bruker, Germany) in dimethyl sulfoxide-d6 (DMSO-d6) using tetramethylsilane as internal standard. Chemical shifts (d) are expressed in ppm and coupling constants (s) singlet, (d) doublet, (t) triplet, (m) multiplet. Position of carbons described in 13C NMR interpretation is as per general structure (Figure 1) for all the compounds except 5a. The infrared spectra of compounds were recorded in KBr on Fourier Transform (FTIR-8400S, Shimadzu, Japan) infrared spectrophotometer. Mass spectra (FAB) were recorded on LC-MS/MS (API-4000 TM, Applied BioSystems, MDS SCIEX, Canada). Elemental analyses were performed on a Perkin-Elmer model 240c analyzer (Perkin Elmer, USA).
Figure 1: General structure for 2-(4-methylbenzenesulphonamido)pentanedioic acid amides
General procedure for synthesis of 2-(4-methylbenzene-sulfonamido)pentanedioic acid amide derivatives (5a-l): 2-(4-Methylbenzenesulfonamido)pentanedioic acid amide derivatives were synthesized based on following procedure.
Synthesis of 4-methylbenzene-1-sulfonyl chloride (2): Toluene (9.2 mL, 0.1 mol) was taken in a 250 mL three necked round bottom flask placed on an ice bath fitted with a mercury sealed mechanical stirrer, a calcium chloride guard tube and a 100 mL dropping funnel. Chlorosulfonic acid (25 mL, 3 equiv., 0.36 mole) was placed in the dropping funnel and was added drop wise slowly by continuous stirring. The temperature of the ice bath was maintained at 0°C. After the addition of the chlorosulfonic acid completely the mixture was allowed to come to room temperature. Then the product was poured slowly into well stirred crushed ice. The crude 4-methylbenzene-1-sulfonyl chloride (2) was filtered and washed with water to remove the excess acid. The crude product was recrystalized from water. The yield was 15.20 g (84.21%) and m.p.: 155-157°C.
Synthesis of 2-(4-methylbenzenesulfonamido)pentanedioic acid (3): This was prepared form 4-methylbenzene-1-sulfonyl chloride (2) and L-(+)-glutamic acid according to the procedure reported below.
L-(+)-Glutamic acid (20 g, 0.13 mole) was taken in a 250 mL conical flask and placed on a water bath, fitted with a magnetic stirrer. Sodium hydroxide solution (2N) was added slowly till all the L-glutamic acid dissolved and the mass became distinctly alkaline to phenolphthalein. The water bath was maintained at 60-80°C and 4-methylbenzene-1-sulfonyl chloride (2; 36.10 g, 0.2 mole) was added slowly with continuous stirring and simultaneous addition of sodium hydroxide solution (2N) to keep the mass alkaline. The reaction was continued till a clear homogeneous solution results. After the reaction was over, it was allowed to cool, acidified to PH paper with concentrated hydrochloric acid, saturated with sodium chloride, extracted with chloroform, and allowed to dry overnight with anhydrous magnesium sulfate. Chloroform was removed to yield the crude 2-(4-methylbenzenesulfonamido)penta nedioic acid (3). The crude product was recrystalized from hot water after charcoal treatment. Yield 57.89%, m.p.: 150-153°C. Rf 0.76. Neutral equivalent (found: 297.20, Calc. For C12H15NO6S: 301). IR (KBr) vmax (cm-1) 3136.95 (C-H str. of phenyl ring), 3059.14-2620.21 (O-H str. of COOH), 1667.16, (C=O str.), 1502.34 (C=C str. of phenyl ring), 1322.65 (C-O str. or O-H def of COOH), 1381.06 (S=O str. antisym of SO2N), 1267.80 (S=O str. sym of SO2N), 825 (out of plane C-H def due to p-subs in phenyl ring), 3294.93 (N-H str.), 1664.16 (N-H bending). 1H NMR (400 MHz, DMSO-d6) d = 7.519 (d, 2H, 2', 6' H of C6H4-CH3), 7.071 (d, 2H, 3', 5' H of C6H4-CH3), 3.7 (t, 3H, -OC-C.H-CH2-), 2.152 (s, 1H, -CH3), 2.14 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6) d = 129.12 (C-1 & 5), 118.14 (C-2 & 4), 143.76 (C-3), 168.32 (C-8), 176.20 (C-18). MS (FAB; m/z): 301. [M+1]. Elemental analysis (C12H15NO6S); calcd. C, 47.83; H, 5.02; N, 4.65; found C, 47.70; H, 4.91; N, 4.59%.
Synthesis of 1((4-methylbenzene)sulfonyl)-5-oxopyrrolidine-2-caroboxylic acid (4): 2-(4-Methylbenzenesulfonamido)pentanedioic acid (3; 32 g, 0.113 mole) was taken in a 500 ml round bottom flask, fitted with a reflux condenser and a calcium chloride guard tube. Acetyl chloride (70 mL) and benzene (40 mL) were added to it and refluxed in a steam bath for four hours. After refluxing, benzene was removed by distillation and the reaction mass was cooled. After that chopped ice poured in the round bottom flask with continuous stirring. It was kept overnight in freeze, when the semisolid mass solidified. It was filtered, washed with distilled water. The crude product was recrystallized from dilute ethanol after charcoal treatment. This was later used in the subsequent steps. Yield: 2.80 g (82.84%); m.p.: 121-123°C.
Synthesis of 2-(4-methylbenzenesulfonamido)pentanedioic acid amide derivatives (5a-l): The 1((4-methylbenzene)sulfonyl)-5-oxopyrrolidine-2-caroboxylic acid (4) was dissolved in dry benzene (10 mL) and the whole mass was cooled by dipping in ice water. Aniline and its derivatives, previously cooled was added to it and mixed well. The whole mass was transferred in a mortar and pestle and triturated when the product was obtained. It was then acidified with dilute hydrochloric acid. The precipitate obtained was filtered and washed with distilled water to remove excess acid. The residue was dried and recrystallized from dilute ethanol after charcoal treatment.
2-(4-Methylphenylsulfonamido)-5-oxo-5-ureidopentanoic acid (5a): White colour solid; yield: 83.10%; m.p.: 115-117°C; Rf 0.61; IR (KBr) vmax (cm-1): 2974.47-2747.27 (O-H str. of COOH), 1641.54 (C=O str.), 1552.24 (C=C str. of phenyl ring), 1347.41 (S=O str. antisym of SO2N), 1314.44 (S=O str. sym of SO2N), 871.61 (out of plane C-H def due to p-subs in phenyl ring), 3374 (N-H str.), 1647 (N-H bending). 1H NMR (DMSO-d6): d 7.54 (d, 2H, 2', 6' H of C6H4-CH3), 7.341 (d, 2H, 3', 5' H of C6H4-CH3), 4.102 (t, 3H, -OC-C.H-CH2-), 2.531 (s, 1H, -CH3), 2.214 (s, 1H, -SO2NH). 13C NMR (DMSO-d6): d 141.89 (C-1), 128.45 (C-2 & 6), 126.03 (C-3 & 5), 139.55 (C-4), 58.37 (C-7), 27.24 (C-8), 36.17 (C-9), 21.58 (C-10), 20.38 (C-18). MS (FAB; m/z): 343. [M+1]. Elemental analysis (C13H17N3O6S); calcd. C, 45.47; H, 4.99; N, 12.24; found C, 45.53; H, 5.05; N, 12.19%.
2-(4-Methylphenylsulfonamido)-5-oxo-5-(phenylamino)pentanoic acid (5b): Greyish colour solid; yield: 73.20%, m.p.: 132-134°C; Rf 0.72; IR (KBr) vmax (cm-1): 2924.42-2674.22 (O-H str. of COOH), 1671.41 (C=O str.), 1545.74 (C=C str. of phenyl ring), 1309.7 (S=O str. antisym of SO2N), 1241.77 (S=O str. sym of SO2N), 871.44 (out of plane C-H def due to p-subs in phenyl ring), 3379 (N-H str.), 1674 (N-H bending). 1H NMR (DMSO-d6): d 7.571 (d, 2H, 2', 6' H of C6H4-CH3), 7.321 (d, 2H, 3', 5' H of C6H4-CH3), 7.212 (d, 2H, 2', 6' H of C6H5), 7.208 (d, 2H, 3', 5' H of C6H5), 3.71 (t, 3H, -OC-C.H-CH2-), 2.541 (s, 1H, -CH3), 2.04 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 140.78 (C-1), 128.24 (C-2 & 6), 128.35 (C-3 & 5), 137.87 (C-4), 57.74 (C-7), 27.05 (C-8), 36.33 (C-9), 120.54 (C-11& 15), 128.87 (C-12 &14), 21.43 (C-18). MS (FAB; m/z): 376. [M+1]. Elemental analysis (C18H20N2O5S); calcd. C, 57.43; H, 5.36; N, 7.44; found C, 57.20; H, 5.15; N, 7.39%.
2-(4-Methylphenylsulfonamido)-5-((4-chlorophenyl)amino)-5-oxopentanoic acid (5c): White colour solid; yield: 56.23%; m.p.: 178-179°C; Rf 0.77; IR (KBr) vmax (cm-1): 2841-2577 (O-H str. of COOH), 1674.74 (C=O str.), 1514.46 (C=C str. of phenyl ring), 1370 (C-N phenyl ring), 1308.51 (S=O str. antisym of SO2N), 1215.74 (S=O str. sym of SO2N), 877 (out of plane C-H def due to p-subs in phenyl ring), 741.7 (C-Cl str. of phenyl ring), 3474.11 (N-H str.), 1541 (N-H bending). 1H NMR (DMSO-d6): d 7.452 (d, 2H, 2', 6' H of C6H4-CH3), 7.312 (d, 2H, 3', 5' H of C6H4-CH3), 7.231 (d, 2H, 2', 6' H of C6H4-Cl), 7.211 (d, 2H, 3', 5' H of C6H4-Cl), 4.14 (t, 3H, -OC-C.H-CH2-), 2.511 (s, 1H, -CH3), 2.40 (q, 4H,-H2C-H2C-), 2.002 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 141.41 (C-1), 129.74 (C-2 & 6), 127.47 (C-3 & 5), 137.77 (C-4), 57.23 (C-7), 27.17 (C-8), 37.33 (C-9), 121.4 (C-11& 15), 127.83 (C-12 &14), 21.47 (C-18). MS (FAB; m/z): 410. [M+1]. Elemental analysis (C18H19ClN2O5S); calcd. C, 52.62; H, 4.66; N, 6.82; found C, 52.64; H, 4.61; N, 6.79%.
2-(4-Methylphenylsulfonamido)-5-((4-bromophenyl)amino)-5-oxopentanoic acid (5d): White colour solid; yield: 71.77%; m.p.: 201-203°C; Rf 0.73; IR (KBr) vmax (cm-1): 3044.41-2741.74 (O-H str. of COOH), 1641.7 (C=O str.), 1541.71 (C=C str. of phenyl ring), 1341 (S=O str. antisym of SO2N), 1235.81 (S=O str. sym of SO2N), 894.4 (out of plane C-H defdue to p-subs in phenyl ring), 741.12 (C-Br str. of phenyl ring), 3574 (N-H str.), 1674 (N-H bending). 1H NMR (DMSO-d6): d 7.532 (d, 2H, 2', 6' H of C6H4-CH3), 7.612 (d, 2H, 3', 5' H of C6H4-CH3), 7.842 (d, 2H, 2', 6' H of C6H4-Br), 7.89 (d, 2H, 3', 5' H of C6H4-Br), 3.41 (t, 3H, -OC-C.H-CH2-), 2.43 (s, 1H, -CH3), 2.0 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 142.8 (C-1), 127.81 (C- 2 & 6), 128.74 (C-3 & 5), 137.88 (C-4), 57.73 (C-7), 28.09 (C-8), 36.37 (C-9), 122.4 (C-11& 15), 128.71 (C-12 &14), 21.03 (C-18). MS (FAB; m/z): 455. [M+1]. Elemental analysis (C18H19BrN2O5S); calcd. C, 47.48; H, 4.21; N, 6.15; found C, 47.43; H, 4.29; N, 6.18%.
2-(4-Methylphenylsulfonamido)-5-((4-nitrophenyl)amino)-5-oxopentanoic acid (5e): Yellow colour solid; yield: 69.87%; m.p.: 151-153°C; Rf 0.70; IR (KBr) vmax (cm-1): 2871.9-2555.41 (O-H str. of COOH), 1674.17 (C=O str.), 1514.9 (C=C str. of phenyl ring), 1281.48 (S=O str. antisym of SO2N), 1211.14 (S=O str. sym of SO2N), 874.94 (out of plane C-H def due to p-subs in phenyl ring), 1581.26 (C-NO2), 3521 (N-H str.), 1647 (N-H bending). 1H NMR (DMSO-d6): d 7.601 (d, 2H, 2', 6' H of C6H4-CH3), 7.542 (d, 2H, 3', 5' H of C6H4-CH3), 7.98 (d, 2H, 2', 6' H of C6H4-NO2), 7.93 (d, 2H, 3', 5' H of C6H4-NO2), 3.52 (t, 3H, -OC-C.H-CH2-), 2.502 (s, 1H, -CH3), 2.1 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 140.59 (C-1), 128.55 (C-2 & 6), 128.83 (C-3 & 5), 137.74 (C-4), 55.14 (C-7), 27.14 (C-8), 36.77 (C-9), 121.54 (C-11& 15), 128.43 (C-12 &14), 21.23 (C-18). MS (FAB; m/z): 421. [M+1]. Elemental analysis (C18H19N3O7S); calcd. C, 51.30; H, 4.54; N, 9.97; found C, 51.38; H, 4.51; N, 9.92%.
2-(4-Methylphenylsulfonamido)-5-((2-chlorophenyl)amino)-5-oxopentanoic acid (5f): Brown colour solid; yield: 68.12%; m.p.: 222-224°C; Rf 0.83; IR (KBr) vmax (cm-1): 2924.42-2568.41 (O-H str. of COOH), 1675 (C=O str.), 1547.41 (C=C str. of phenyl ring), 1342.45 (S=O str. antisym of SO2N), 1244.12 (S=O str. sym of SO2N), 843.31 (out of plane C-H defdue to p-subs in phenyl ring), 741.41 (C-Cl str. of phenyl ring), 3247 (N-H str.), 1684 (N-H bending). 1H NMR (DMSO-d6): d 7.385 (d, 2H, 2', 6' H of C6H4-CH3), 7.219 (d, 2H, 3', 5' H of C6H4-CH3), 7.256 (d, 2H, 3', 5' H of C6H4-Cl), 4.11 (t, 3H, -OC-C.H-CH2-), 2.421 (s, 1H, -CH3), 2.32 (q, 4H,-H2C-H2C-), 2.04 (s, 1H, -SO2NH-).13C NMR (DMSO-d6): d 142.8 (C-1), 129.24 (C-2 & 6), 128.84 (C-3 & 5), 137.53 (C-4), 57.79 (C-7), 27.11 (C-8), 36.73 (C-9), 120.72 (C-11& 15), 129.17 (C-12 &14), 21.47 (C-18). MS (FAB; m/z): 410. [M+1]. Elemental analysis (C18H19ClN2O5S); calcd. C, 52.62; H, 4.66; N, 6.82; found C, 52.59; H, 4.68; N, 6.81%.
2-(4-Methylphenylsulfonamido)-5-((2-bromophenyl)amino)-5-oxopentanoic acid (5g): White colour solid; yield: 78.21%; m.p.: 250-252°C; Rf 0.64; IR (KBr) vmax (cm-1): 3012.54-2753.66 (O-H str. of COOH), 1570.8 (C=O str.), 1527.31 (C=C str. of phenyl ring), 1347.5 (S=O str. antisym of SO2N), 1260.48 (S=O str. sym of SO2N), 840.21 (out of plane C-H def due to p-subs in phenyl ring), 741.7 (C-Br str. of phenyl ring), 3287.22 (N-H str.), 1623 (N-H bending). 1H NMR (DMSO-d6): d 7.714 (d, 2H, 2', 6' H of C6H4-CH3), 7.524 (d, 2H, 3', 5' H of C6H4-CH3), 7.759 (s, 1H, 6' H of C6H4-Br), 7.421 (d, 2H, 3', 5' H of C6H4-Br), 3.24 (t, 3H, -OC-C.H-CH2-), 2.42 (s, 1H, -CH3), 2.02 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 142.07 (C-1), 128.52 (C-2 & 6), 128.81 (C-3 & 5), 137.43 (C-4), 57.79 (C-7), 27.09 (C-8), 36.73 (C-9), 121.34 (C-11& 15), 128.66 (C-12 &14), 21.72 (C-18). MS (FAB; m/z): 455. [M+1]. Elemental analysis (C18H19BrN2O5S); calcd. C, 47.48; H, 4.21; N, 6.15; found C, 47.45; H, 4.18; N, 6.10%.
2-(4-Methylphenylsulfonamido)-5-((2-nitrophenyl)amino)-5-oxopentanoic acid (5h): Greyish colour solid; yield: 61.25%; m.p.: 210-212°C; Rf 0.88; IR (KBr) vmax (cm-1): 2974.45-2695.67 (O-H str. of COOH), 1670.7 (C=O str.), 1573.41 (C=C str. of phenyl ring), 1310.3 (S=O str. antisym of SO2N), 1223.78 (S=O str. sym of SO2N), 851.71 (out of plane C-H def due to p-subs in phenyl ring), 1571.41 (C-NO2), 3344 (N-H str.), 1671 (N-H bending). 1H NMR (DMSO-d6): d 7.71 (d, 2H, 2', 6' H of C6H4-CH3), 7.574 (d, 2H, 3', 5' H of C6H4-CH3), 8.01 (s, 1H, 6' H of C6H4-NO2), 8.012 (d, 2H, 3', 5' H of C6H4-NO2), 3.7 (t, 3H, -OC-C.H-CH2-), 2.71 (s, 1H, -CH3), 2.1 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 141.7 (C-1), 128.84 (C-2 & 6), 128.32 (C-3 & 5), 134.87 (C-4), 57.43 (C-7), 27.12 (C-8), 39.23 (C-9), 122.43 (C-11& 15), 128.42 (C-12 &14), 21.47 (C-18). MS (FAB; m/z): 421. [M+1]. Elemental analysis (C18H19N3O7S); calcd. C, 51.30; H, 4.54; N, 9.97; found C, 51.33; H, 4.49; N, 9.90%.
2-(4-Methylphenylsulfonamido)-5-((3-chlorophenyl)amino)-5-oxopentanoic acid (5i): Brown colour solid; yield: 51.55%; m.p.: 198-200°C; Rf 0.79; IR (KBr) vmax (cm-1): 2947.24-2644.1 (O-H str. of COOH), 1675.47 (C=O str.), 1512.71 (C=C str. of phenyl ring), 1371 (S=O str. antisym of SO2N), 1254.12 (S=O str. sym of SO2N), 847.14 (out of plane C-H def due to p-subs in phenyl ring), 741.7 (C-Cl str. of phenyl ring), 3372 (N-H str.), 1647 (N-H bending). 1H NMR (DMSO-d6): d 7.451 (d, 2H, 2', 6' H of C6H4-CH3), 7.274 (d, 2H, 3', 5' H of C6H4-CH3), 7.684 (d, 2H, 2', 6' H of C6H4-Cl), 7.008 (s, 1H, 5' H of C6H4-Cl), 4.12 (t, 3H, -OC-C.H-CH2-), 2.442 (s, 1H, -CH3), 2.21 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 140.49 (C-1), 129.4 (C-2 & 6), 128.69 (C-3 & 5), 137.33 (C-4), 57.47 (C-7), 26.05 (C-8), 36.42 (C-9), 120.53 (C-11& 15), 128.7 (C-12 &14), 21.22 (C-18). MS (FAB; m/z): 410. [M+1]. Elemental analysis (C18H19ClN2O5S); calcd. C, 52.62; H, 4.66; N, 6.82; found C, 52.59; H, 4.70; N, 6.87%.
2-(4-Methylphenylsulfonamido)-5-((3-bromophenyl)amino)-5-oxopentanoic acid (5j): White colour solid; yield: 48.49%; m.p.: 181.183°C; Rf 0.84; IR (KBr) vmax (cm-1): 2924.12-2541.4 (O-H str. of COOH), 1679 (C=O str.), 1524.41 (C=C str. of phenyl ring), 1340.05 (S=O str. antisym of SO2N), 1214.02 (S=O str. sym of SO2N), 871.11 (out of plane C-H def due to p-subs in phenyl ring), 747.7 (C-Br str. of phenyl ring), 3244.85 (N-H str.), 1641 (N-H bending). 1H NMR (400 MHz, DMSO-d6): d 7.421 (s, 1H, 6' H of C6H4-CH3), 7.232 (d, 2H, 3', 5' H of C6H4-CH3), 7.897 (d, 2H, 2', 6' H of C6H4-Br), 7.481 (s, 1H, 5' H of C6H4-Br), 4.14 (t, 3H, -OC-C.H-CH2-), 2.341 (s, 1H, -CH3), 2.34 (q, 4H,-H2C-H2C-), 2.12 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 140.63 (C-1), 128.78 (C-2 & 6), 128.57 (C-3 & 5), 137.63 (C-4), 57.24 (C-7), 27.06 (C-8), 36.23 (C-9), 120.85 (C-11& 15), 128.96 (C-12 &14), 21.73 (C-18). MS (FAB; m/z): 455. [M+1]. Elemental analysis (C18H19BrN2O5S); calcd. C, 47.48; H, 4.21; N, 6.15; found C, 47.54; H, 4.15; N, 6.21%.
2-(4-Methylphenylsulfonamido)-5-((3-nitrophenyl)amino)-5-oxopentanoic acid (5k): Yellow colour solid; yield: 41.44%; m.p.: 216-218°C; Rf 0.66; IR (KBr) vmax (cm-1): 3050.44-2741.1 (O-H str. of COOH), 1571.12 (C=O str.), 1524.12 (C=C str. of phenyl ring), 1347.5 (S=O str. antisym of SO2N), 1281.17 (S=O str. sym of SO2N), 884.05 (out of plane C-H def due to p-subs in phenyl ring), 1581.77 (C-NO2), 3295.13 (N-H str.), 1618 (N-H bending). 1H NMR (400 MHz, DMSO-d6): d 7.731 (d, 2H, 2', 6' H of C6H4-CH3), 7.414 (d, 2H, 3', 5' H of C6H4-CH3), 7.971 (d, 2H, 2', 6' H of C6H4-NO2), 7.498 (s, 1H, 5' H of C6H4-NO2), 3.43 (t, 3H, -OC-C.H-CH2-), 2.414 (s, 1H, -CH3), 2.07 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 140.57 (C-1), 127.23 (C-2 & 6), 128.53 (C-3 & 5), 137.85 (C-4), 57.22 (C-7), 27.32 (C-8), 36.74 (C-9), 120.35 (C-11& 15), 128.83 (C-12 &14), 20.46 (C-18). MS (FAB; m/z): 421. [M+1]. Elemental analysis (C18H19N3O7S); calcd. C, 51.30; H, 4.54; N, 9.97; found C, 51.25; H, 4.48; N, 9.89%.
2-(4-Methylphenylsulfonamido)-5-((4-methylphenyl)amino)-5-oxopentanoic acid (5l): White colour solid; yield: 77.12%; m.p.: 270-272°C; Rf 0.82; IR (KBr) vmax (cm-1): 2941.41-2686.12 (O-H str. of COOH), 1641.7 (C=O str.), 1573.42 (C=C str. of phenyl ring), 1318.04 (S=O str. antisym of SO2N), 1214.09 (S=O str. sym of SO2N), 877.14 (out of plane C-H def due to p-subs in phenyl ring), 3373 (N-H str.), 1677.4 (N-H bending). 1H NMR (400 MHz, DMSO-d6): d 7.721 (d, 2H, 2', 6' H of C6H4-CH3), 7.533 (d, 2H, 3', 5' H of C6H4-CH3), 7.271 (d, 2H, 2', 6' H of C6H4-Methyl), 7.21 (d, 2H, 3', 5' H of C6H4-Methyl), 3.62 (t, 3H, -OC-C.H-CH2-), 2.417 (s, 1H, -CH3), 2.14 (s, 1H, -SO2NH-). 13C NMR (DMSO-d6): d 142.61 (C-1), 128.32 (C-2 & 6), 128.68 (C-3 & 5), 137.56 (C-4), 57.74 (C-7), 27.07 (C-8), 36.38 (C-9), 120.72 (C-11& 15), 129.87 (C-12 &14), 21.6 (C-18). MS (FAB; m/z): 390. [M+1]. Elemental analysis (C19H22N2O5S); calcd. C, 58.45; H, 5.68; N, 7.17; found C, 58.49; H, 5.60; N, 7.21%.
In vitro cytotoxic assay: Human breast cancer (MCF-7), Leukemia (K-562), Ovarian cancer (OVCAR-3), Human colon adeno carcinoma (HT-29) and Human kidney carcinoma (A-498) tumor cells were obtained from National Centre for Cell Sciences (Pune, India). The cultures were maintained in Dulbecco's Modified Eagles Medium (DMEM) containing 10% heat inactivated fetal bovine serum (FBS), penicillin (100 units/mL) and streptomycin (100 µg/mL) at 37°C in 5% CO2. Cells were grown in 25 cm2 tissue cultures flask until confluent and used for cytotoxicity assays.
MTT assay: The MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) assay developed by Mosmann (Mosmann, 1983) was modified and used to determine the inhibitory effects of test compounds on cell growth in vitro. In brief, the trypsinized cells from T-25 flask were seeded in each well of 96-well flat bottomed tissue culture plate at a density of 5 x 103 cells/well in growth medium and cultured at 37°C in 5% CO2 to adhere. After 48 hours incubation, the supernatant was discarded and the cells were pretreated with growth medium and were subsequently mixed with different concentrations of both standard (tamoxifen) and test compounds 5a-l (8, 16, 32, 64, 128 and 256 µg/mL) in triplicates to achieve a final volume of 100 µL and then cultured for 48 hours. The compound was prepared as 1.0 mg/mL concentration stock solutions in PBS for al the cell lines. Culture medium and solvent are used as controls. Each well then received 5 µL of fresh MTT (0.5 mg/mL in PBS) followed by incubation for 4 hours at 37°C in 5% CO2. The supernatant growth medium was removed from the wells and replaced with 100 µL of DMSO to solubilize the colored formazan product. The absorbance (Dantu et al., 2012) of the culture plate was read at a wavelength of 570 nm on an ELISA reader, Anthos 2020 spectrophotometer. Both standard and test maintained in triplicate. The IC50 value refers to the drug concentration that produces a 50% reduction in cellular growth when compared to untreated control cells was determined by plotting a graph of Log (concentration of compound) vs % cell inhibition. A line drawn from 50% value on the Y axis meets the curve and interpolate to the X axis. The X axis value gives the Log (concentration of compound). The antilog of that value gives the IC value (Holbeck, 2004).
In vivo anticancer assay: The animal experiments were performed following the approval of study protocols by the Institutional Animal Ethics Committee (BCRCP/IAEC/7/2012). The synthesized compounds were biologically screened against Ehrlich ascites carcinoma (EAC) in female Swiss Albino mice using tumor weight and cell count as activity parameters. Amongst various evaluation systems in vogue, this method has been standardized and numbers of screening results have been reported earlier (De and Pal, 1975; De and Pal, 1977; Ray and De, 2009). Two groups of Swiss Albino mice, each containing five healthy mice of the same sex (female in this case), approximately of same age and body weight (18-20 g), were selected at random and kept in two different cages under identical condition. One of these two groups served as control while the other as test. Ehrlich ascites carcinoma (EAC) cells were collected from the donor mice and were suspended in sterile isotonic solution (0.9% w/v NaCl). The numbers of tumor cells per ml of this suspension were counted under microscope with the help of haemocytometer. A definite number (about 2 x 106/0.2 mL) of these living viable cells was injected or implanted into the peritoneal cavity of each mouse. In this instance, the tumor cells multiplied relatively freely with in the peritoneal cavity and ascites developed. A day of incubation was allowed to establish the disease in the body before the administration of synthesized compounds (Eckhardt et al., 1982). From the second day of transplantation up to the eight day a suitable dose (0.2 mmol/kg body weight) of the drug solution/suspension in sterile phosphate buffer (pH 7.2) was injected intraperitoneally to each mouse in the test group at 24 hours interval. Thus, seven doses of the drug were administered to each mouse in the test group. On the ninth day food and water were withheld or withdrawn 6 hours before the testing operation started. The weight of all the animals was recorded before they were sacrificed. The peritoneal cavity was dissected and the ascites fluid was drawn by a syringe to a suitable volume with sterile ice cold saline and preserved in ice bath. The total number of living cells ml-1 in the peritoneal fluid of the three mice in a group was calculated. The fluid was sucked by absorbent cotton. The weight of the five mice after sacrifice was recorded. The evaluation of the test drug was made by comparing the cell count and ascetic fluid weight of the test with that of the control. The percentage inhibition of ascetic cell count and ascetic fluid weight was obtained by the following expressions.
Percentage inhibition of ascitic cell = (1 - T/C) x 100
Percentage inhibition of ascitic fluid = (1 - T/C) x 100
Where, T = Average No. of ascitic cells per mL in test animals, C= Average No. of ascitic cells per mL in control animals, T = Average weight of ascitic fluid in test animals and C = Average weight of ascitic fluid in control animals
Mitomycin-C (1 mg/kg body weight) in sterile phosphate buffer (pH 7.2) was used as standard.
Statistical analysis: Values are expressed as mean ± SEM and data was analyzed by ANOVA followed by Dunnett's test. P<0.05 was considered as significant.
Result and Discussion
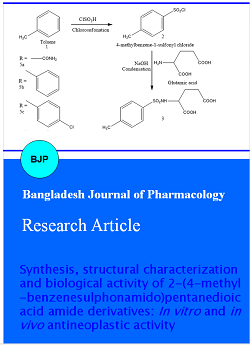
The synthetic strategies followed for the preparation of the substituted 2-(4 methylbenzenesulfonamido) pentanedioic acid bis amide derivatives 5a-l are depicted in Scheme 1. Synthesis was started with chlorosulphonation of toluene, to get 4-methylbenzene-1-sulfonyl chloride 2. This sulphonyl halide proved to be versatile synthon in the subsequent step in the preparation of substituted sulphonyl glutamic acids. With the application of Schotton-Bauman reaction, substituted sulphonyl glutamic acids 3 were prepared by one-step condensation of 4-methylbenzene-1-sulfonyl chloride 2 with L-glutamic acid. In this reaction alkaline medium was maintained to remove the hydrochloric acid which was formed during condensation. Reaction of the resulting intermediate sulphonyl glutamic acids 3 with acetyl chloride afforded cyclized acid intermediates substituted sulphonyloxopyrrolidine carboxylic acids 4. Aminolysis of the intermediates 4 with various amines afforded the corresponding glutamines 5a-l.
Scheme 1: Synthesis of 2-(4-methylbenzenesulfonamido)pentanedioic acid amide derivatives
Formation of 2-(4-methylbenzenesulphonamido)pentanedioic acid amide derivatives 5a-l were confirmed by recording their IR, NMR, mass spectra and elemental analyses. The IR spectra of compounds 5a-l revealed the presence of absorption bands from 3012.54 to 2542 cm-1 that indicate the presence of OH str. of COOH, from 1696.07 to 1570.8 cm-1 for C=O str., from 3287.32 to 3397 cm-1 for N-H str., from 1593-1645 cm-1 for N-H bending, from 1280.84-1354.5 cm-1 for S=O str. antisymmetric of SO2N and 1209-1310.18 cm-1 for S=O str. symmetric of SO2N vibrations. In addition to proton signals of the functional groups and both aromatic ring present in the respective molecule 1H NMR spectra of these compounds contained one proton singlet from d 2.0 to 2.203 ppm which was assigned to -SO2NH- proton, from 2.21 to 2.604 ppm for -CH3 proton and doublets from d 7.201 to 7.98 ppm for aromatic protons confirming the formation of compounds 5a-l. The 13C NMR spectra of compounds 5a-l showed peaks from d 118.57 ppm to 156.38 ppm for aromatic protons, from d 20.12 ppm to 22.31 ppm for -CH3 carbon confirming the formation of compounds 5a-l. The mass spectra of compounds 5a-l showed molecular ion peaks [M+1] at m/z corresponding to their respective molecular masses, which is in consistency with their respective molecular formulas. For the compound 5a, molecular weight 343 is consistent with the molecular formula C13H17N3O6S. The values for the remaining compounds have been presented under the experimental part.
The cytotoxicity of the synthesized compounds 5a-l and the intermediate compound 3 were studied using the MTT assay in five human cancer cell lines. The results are listed in Table I. Substitution of various moieties such as Cl, Br, nitro and methyl were done at different positions of the phenyl ring to study the electronic effects of the related substituents. Bromo substitution showed the best anticancer properties against all the cell line at position 4 (para) and 3 (meta) of the phenyl ring (compound 5d and 5j). Comparison of cytotoxic effects of nitro derivatives in all cell lines exhibited that para position is the best position for rendering of the optimal activity (compound 5e). Investigation of the role of methyl group at position para caused high cytotoxic effects for compound 5l. Insertion of the phenyl ring without any moiety resulted in implausible effects in all cell lines. Compounds 5b, 5g, 5h and 5k showed low cytotoxic effects on all the cell lines whereas compounds 3, 5a, 5d, 5e, 5j and 5l showed high cytotoxicity in all cell lines with IC50 concentrations lines, except for the Human colon adeno carcinoma (HT-29) cell line as compare to standard drug, tamoxifen. The primary antitumor activity of tamoxifen by inhibition protein kinase C (Gelman, 1997) and also ability to facilitate the apoptosis in cancer cell not expressing estrogen receptor is due to generation of oxidative stress resulting in thiol depletion and activation of the transcriptional factor NF-kappaB. Many clinical studies explain the tamoxifen application in various kinds of maligamant diseases (Ferlini et al., 1999; NCI, 1999).
Table I: In vitro anticancer activity (IC50) of synthesized compounds against human breast cancer (MCF-7), leukemia (K-562), ovarian cancer (OVCAR-3), human colon adenocarcinoma (HT-29) and human kidney carcinoma (A-498)
| Compound | Human breast cancer (MCF-7) |
Leukemia (K-562) |
Ovarian cancer (OVCAR-3) |
Human colon adenocarcinoma (HT-29) |
Human kidney carcinoma (A-498) |
|---|---|---|---|---|---|
| 3 | 14.7 ± 0.0a | 13.2 ± 0.0a | 13.4 ± 0.0a | 26.4 ± 0.0a | 24.5 ± 0.0a |
| 5a | 12.2 ± 0.0a | 11.2 ± 0.0a | 21.3 ± 0.0a | 40.2 ± 0.1a | 25.3 ± 0.0a |
| 5b | 81.3 ± 0.0a | 67.2 ± 0.0a | 58.3 ± 0.1a | 71.2 ± 0.0a | 70.1 ± 0.1a |
| 5c | 88.2 ± 0.4 | 54.2 ± 0.2 | 87.2 ± 0.4 | >100 | 77.2 ± 0.4 |
| 5d | 14.2 ± 0.0a | 20.1 ± 0.0a | 19.2 ± 0.0a | 35.3 ± 0.1a | 23.2 ± 0.1a |
| 5e | 17.2 ± 0.0a | 15.2 ± 0.0a | 11.4 ± 0.0a | 29.3 ± 0.0a | 26.2 ± 0.0a |
| 5f | 78.3 ± 0.5 | 98.3 ± 0.4 | 69.3 ± 0.4 | >100 | 88.3 ± 0.2 |
| 5g | 70.3 ± 0.0a | 87.3 ± 0.0a | 58.4 ± 0.0a | 88.9 ± 0.0a | 82.4 ± 0.0a |
| 5h | 74.2 ± 0.3 | 72.0 ± 0.3 | 65.3 ± 0.4 | >100 | 70.2 ± 0.4 |
| 5i | 88.2 ± 0.0a | 98.3 ± 0.0a | 80.2 ± 0.0a | 99.3 ±0.0a | 71.2 ± 0.0a |
| 5j | 10.2 ± 0.0a | 18.2 ± 0.0a | 24.3 ± 0.0a | 36.2 ± 0.0a | 19.2 ± 0.0a |
| 5k | 87.2 ± 0.0a | 73.2 ± 0.0a | 81.0 ± 0.0a | 91.3 ± 0.1a | 41.2 ± 0.0a |
| 5l | 14.2 ± 0.0a | 16.2 ± 0.0a | 17.1 ± 0.0a | 28.3 ± 0.1a | 23.2 ± 0.0a |
| Tamoxifen (standard) |
8.2 ± 0.0a | 10.4 ± 0.0a | 9.3 ± 0.0a | 14.3 ± 0.0a | 12.2 ± 0.0a |
| Compounds with IC50>100 µM were considered not active; ap<0.05 compared to tamoxifen (standard) | |||||
All the newly synthesized final compounds 5a-l along with compound 3 was screened for their anticancer activity against Ehrlich ascites carcinoma is summarized in Table II together with standard drug mitomycin-C. Among the synthesized compounds, the ureide 5a, p-bromoanilide 5d, p-nitoanilide 5e, o-bromoanilide 5j and p-methylanilide 5l derivatives showed encouraging activity in both the parameter, viz., ascetic fluid weight (93.85 % for 5a, 91.48 % for 5d, 95.01 % for 5e, 95.97 % for 5j and 96.37 % for 5l) and ascetic cell count (98.95 % for 5a, 98.31 % for 5d, 95.80 % for 5e, 96.84 % for 5j and 94.16 % for 5l) respectively as compare to mitomycin-C (100% in both the parameters). Hence, a detailed and prolonged study is necessary to establish their activity in other models.
Table II: In vivo anticancer activity of synthesized compounds against Ehrlich ascites carcinoma
| Copound | AA | BB | CC | DD | EE | FF | GG |
|---|---|---|---|---|---|---|---|
| 3 | 5 | 72.5 | 7.1 ± 0.1a | 90.3 | 2.83 | 0.7 ± 0.1a | 76.2 |
| 5a | 5 | 72.5 | 0.8 ± 0.0a | 99.0 | 2.83 | 0.2 ± 0.0a | 93.9 |
| 5b | 5 | 72.5 | 10.2 ± 0.0a | 62.3 | 2.83 | 1.0 ± 0.1a | 64.0 |
| 5c | 5 | 72.5 | 13.0 ± 0.3 | 82.1 | 2.83 | 1.1 ± 0.3 | 60.8 |
| 5d | 5 | 72.5 | 1.2 ± 0.1a | 98.3 | 2.83 | 0.2 ± 0.1a | 91.5 |
| 5e | 5 | 72.5 | 3.0 ± 0.0a | 95.8 | 2.83 | 0.1 ± 0.0a | 95.0 |
| 5f | 5 | 72.5 | 11.5 ± 0.3 | 84.1 | 2.83 | 1.6 ± 0.3 | 42.4 |
| 5g | 5 | 72.5 | 10.0 ± 0.1a | 86.3 | 2.83 | 1.4 ± 0.0a | 50.2 |
| 5h | 5 | 72.5 | 14.2 ± 0.2 | 80.4 | 2.83 | 0.9 ± 0.6 | 69.2 |
| 5i | 5 | 72.5 | 11.3 ± 0.0a | 84.4 | 2.83 | 1.0 ± 0.0a | 66.4 |
| 5j | 5 | 72.5 | 2.3 ± 0.0a | 96.8 | 2.83 | 0.1 ± 0.0a | 96.0 |
| 5k | 5 | 72.5 | 14.8 ± 0.1a | 79.6 | 2.83 | 0.9 ± 0.1a | 67.9 |
| 5l | 5 | 72.5 | 4.2 ± 0.0a | 94.2 | 2.83 | 0.1 ± 0.0a | 96.4 |
| Mitomycin-C (standard) |
5 | 72.5 | 0.0 | 100.0 | 2.83 | 0.0 | 100.0 |
| AA= Number of animals in each group; BB= Average No. of ascitic cells per ml in control, C (X106 cells/mL); CC=Average No. of ascitic cells per ml in test ± SEM, T (X 106 cells/mL); DD=Percent inhibition of ascitic cells (1-T/C) X 100; EE=Average weight of ascitic fluid in control C (g); FF= Average weight of ascitic fluid in test ± SEM, T (g); GG= Percent inhibition of ascitic fluid (1-T/C) X 100; *p<0.05 compared to mitomycin-C (standard) | |||||||
Conclusion
Thus, 12 new compounds along with an intermediate compound 3 are synthesized, characterized and biologically screened for in vitro and in vivo antitumor activity. It is noticed that final derivative compounds showing better activity than the parent compound and it may be due to the substituents present in those compounds.
References
Dantu AS, Shankarguru P, Ramya D D, Vedha HBN. Evaluation of in vitro anticancer activity of hydroalcoholic extract of Tabernaemontana divaricata. Asian J Pharmaceut Clin Res. 2012; 5: 59-61.
De AU, Pal D. Possible antineoplastic agents I. J Pharm Sci. 1975; 64: 262-66.
De AU, Pal D. Possible antineoplastic agents II. J Pharm Sci. 1977; 66: 232-35.
Debnath B, Srikanth K, Banerjee S, Jha T. 1,5-N,N´-Disubstituted-2-(substituted benzenesulphonyl)-glutamamides as antitumor agents. Part 2. Synthesis, biological activity and QSAR study. Internet Electron J Mol Des. 2002; 1: 488-502.
Eckhardt AE, Malone BN, Goldstein IJ. Inhibition of Ehrlich ascites tumor cell growth by Griffonia simplicifolia I Lectin in vivo. Cancer Res. 1982; 42: 2977-79.
Eidinoff ML. Pyrimidine studies: I. Effect of DON (6-Diazo-5-oxo-L-norleucine) on incorporation of precursors into nucleic acid pyrimidines. Cancer Res. 1958; 18: 105-09.
Ferlini C, Scambia G, Marone M, Distefano M, Gaggini C, Ferrandina G, Fattotossi A, Isola G, Benedetti Panici P, Mancuso S. Tamoxifen induces oxidative stress and apotosis in oestrogen receptor-negative human cancer cell line. Br J Cancer. 1999; 79: 257-63.
Gelman EP. Tamoxifen for the treatment of malignancies other breast and endometrial carcinoma. Semin Oncol. 1997; Suppl 1.24: 165-70.
Graff S, Rittenberg D and Foster GL. The glutamic acid of malignant tumors. J Biol Chem. 1940; 133: 745-52.
Hartman SC. Metabolic pathways. New York, Academic Press, 1970, pp 1-5.
Holbeck SL. Update on NCI in vitro drug screen utilities. Eur J Cancer. 2004; 40: 785-93.
Keren R, Stark AA. Gamma-glutamyl transpeptidase-dependent mutagenicity and cytotoxicity of gamma-glutamyl derivatives: A model for biochemical targeting of chemotherapeutic agents. Environ Mol Mutagen. 1998; 32: 377-86.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65: 55-63.
NCI. PDQ Clinical trial Search Bethesda. USA, National Cancer Institute, 1999.
Petit GR. Biosynthetic products for cancer chemotherapy. New York, Plenum Press, 1977. pp 158-62.
Ray S, De AU. Synthesis and biological activity of succinimidobenzenesulfonyl oxopyrrolidine analogs as possible antineoplastic agent. Asian J Chem. 2009; 21: 379-87.
Rodwell VW. Metabolism of purine and pyrimidine nucleotides. 25th ed. Stamford, Connecticut, Appleton and Lange, 2000, pp 386-92.
Rosowsky A, Wick MM, Kin SH. Structural analogs of L-glutamic acid-(4-hydroxyanalide) and -(3,4-dihydroxyanalide) as potential agents against melanoma. J Med Chem. 1979; 22: 1034-37.