In vitro antimicrobial evaluation of pyrazolone derivatives
Abstract
In this investigation, unsubstituted pyrazolin-3(5)-ones (MePzO= 3-methyl-5-pyrazolone, PrPzO= 3-propyl-5-pyrazolone and PhPzO= 3-phenyl-5-pyrazolone) have been synthesized. The synthesized compounds were also screened for their antibacterial activity against some Gram-positive and Gram-negative bacteria using agar well diffusion method. The compounds with different concentrations were prepared and the activity was determined by measuring the diameter of the inhibition zone (in mm). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the compounds were determined against six bacteria. The antibacterial activities were influenced functional groups (methyl, propyl and phenyl). PhPzO exhibited the strongest activity (MIC, 0.625 mg/mL; MBC, 2.5 mg/mL) against Bacillus subtilis.
Introduction
Pyrazolin-5-ones are well known nitrogen containing five membered heterocyclic compounds. They have been used as therapeutic agents, such as analgesic, antipyretic, anti-inflammatory (Brogden, 1986; Gursoy et al., 2000; Ratnadeep et al., 2010; El-Hawash et al., 2006; Chao et al., 2008), antioxidant (Manojkumar et al., 2009; Watanabe et al., 1994; Ramana Kumar et al., 2012), antiproliferative (Kim et al., 2005), antibacterial (Al-Haiza et al., 2001; Raman et al., 2004), antifungal activities (Vincent, 1999; Ramaraj et al., 2010) and is useful in the treatment of a variety of disorders caused by Human Immunodeficiency Virus (HIV) (Hadi et al., 2010). Pyrazolone derivatives, such as antipyrine (phenazone), 4-aminopyrine (aminophenazone), metamizol (novalgin), and 4-isopropylpyrine (propyphenazone; PP) are analgesic substances (Himly et al., 2003) and edaravone (3-methyl-1-phenyl-2-pyrazoline-5-one) is found as a drug for brain ischemia, myocardial ischemia, treatment of fatal neurodegenerative diseases and cardiovascular diseases (Tsujita et al., 2004; Higashi et al., 2006; Zhang et al., 2012; Kikuchi et al., 2010).
In view of these observations, we report the synthesis of pyrazolone derivatives, and their antimicrobial evaluation and investigate the effects of concentrations of the compounds on the bacterial strains. The derivatives show good inhibition towards some bacteria.
Materials and Methods
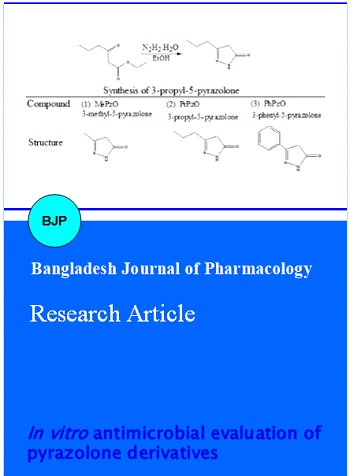
As a part of this study for vibrational spectra, we analyzed the infrared spectra recorded on a FT Bruker Vector 22 infrared spectrophotometer using pressed KBr disk. The melting point was determined using a BarnStead (BI 9300) electrothermal apparatus. Starting materials ethyl acetoacetate, ethyl butyrylacetate and ethyl benzoylacetate were purchased from Acros. Hydrazine monohydrate and ethanol were purchased from Merck. The solvent was dried before use by the standard methods (Perrin, 1988). The preparation of pyrazolone derivatives involve the condensation of a beta-keto ester with substituted or unsubstituted hydrazines. The compounds were synthesized via a similar method described elsewhere (Khalil et al., 2005; Makhija et al., 2004; Song and Zhu, 2001; Abood and Al-Shlhai, 2012). The synthesis of 3-propyl-5-pyrazolone and the structures of the compounds in this investigation are shown in Scheme 1.
Scheme 1: The synthesis of 3-propyl 5 pyrazolone and the structures of the compounds
Synthesis of the 3-methyl-5-pyrazolone (1), 3-propyl-5-pyrazolone (2) and 3-phenyl-5-pyrazolone (3): 3-Methyl-5-pyrazolone [from ethyl acetoacetate with hydrazine monohydrate in hot ethanol, Yield: 82%. m.p.: 202-203°C; FTIR (KBr, cm-1): 3400 (v…N-H), 2300-3242 (v…C-H, m), 1678 (v…C=O, s), 1609 (v…C=N, m), 956 (N-N, w)]; 3-propyl-5-pyrazolone [from ethyl butyrylacetate with hydrazine monohydrate in hot ethanol, Yield: 88 %; m.p.: 207-209 °C; FTIR (KBr, cm-1): 3165 (v…N-H), 2344-2959 (v…C-H, m), 1620 (v…C=O, s), 1599 (v…C=N, m), 901 (N-N, w)]; and 3-phenyl-5-pyrazolone [from ethyl benzoyl-acetate with hydrazine monohydrate in hot ethanol, Yield: 90%; m.p.: 240-241°C; FTIR (KBr, cm-1): 3448 (v…N-H), 2300-3121 (v…C-H, m), 1624 (v…C=O, s), 1491 (v…C=N, m), 930 (N-N, w)] were prepared.
Antimicrobial assay
The antibacterial test of the synthesized compounds (1, 2 and 3) was carried out using the agar well diffusion method (Chohan et al., 2003) to determine the minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs).
The synthesized compounds were screened for their antibacterial activities against six bacterial strains of three Gram-negative bacteria Escherichia coli (PTCC 1270), Salmonella enterica (PTCC 1709), Pseudomonas aeruginosa (PTCC 1430) and three Gram-positive bacteria Staphylococcus aureus (PTCC 1112), Enterococcus faecalis (PTCC 1237), Bacillus subtilis (PTCC 1254) by the agar well diffusion method (Table I) (Chohan et al., 2003). Tetracycline (TE 30), was used as standard for antibacterial activity. All the microbial cultures were adjusted to 0.5 Mc Farland standards (equivalent turbidity 1/5 x 108 CFU/mL). 20 mL of Mueller Hinton Agar medium was poured into each petri plate (12 cm) and plates were swabbed with 100 uL inocula of the test microorganisms and kept for 15 min for adsorption. The compounds to be tested for activity were dissolved in dimethylsulfoxide. In the first step, ten milligrams of the compounds were dissolved in 1 mL of DMSO. Using sterile cork borer of 6 mm diameter, wells were bored into the seeded agar plates and these were loaded with a 100 mL volume with concentration of 10.0-0.039 mg/mL. All the plates were incubated at 37°C for 24 hours and the inhibition zone diameters around each disk were measured and recorded in millimeters and presented in Table I. MIC values against these organisms were determined by serial dilution method using DMSO. MIC determination was performed in a 96-well microtiter plate. For MIC assay, a row of 12 wells was used. In this method, the various test concentrations of synthesized compounds were prepared from 10 to 0.039 mg/mL (No. 1 to 9) and was labeled 10-0.039 mg/mL. 100 uL sterile Mueller Hinton Broth (MHB) was poured in the wells from 2-12 followed by addition of 100 μL test compound in well 1, 2 and 12 containing 10 mg/mL concentration. In this way a range of two-fold serial dilution were prepared (10-0.039 mg/mL) by performing two-fold serial dilution from well 2 to the well 9. To each well, 100 μL of standard inoculums (1.5 x 108 CFU/mL) was added from well 1 to the well 10. Then 10 uL resazurin was added in each of the wells. Turbidity was observed after incubating the inoculated wells at 37°C for 24 hours. The last dilution with no turbidity was considered as MIC. Then from all wells were cultured on plates to determine the minimum lethal bacterial concentration (MBC). Then plates were incubated at 37°C for 24 hours. The well containing the lowest concentration with lack of bacterial growth was visible as MBC. Both MIC and MBC results are presented in Table II.
Table I: Inhibition of bacterial growth by using different concentration of the compounds
| Bacterium | S. aureus | E. faecalis | B. subtilis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard drug | 25 | 25 | 25 | ||||||
| Concentration (mg/mL) | 10 | 5 | 2.5 - 0.04 | 10 | 5 | 2.5-0.04 | 10 | 5 | 2.5-0.04 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 |
| 2 | NT | 13 | 0 | NT | 0 | 0 | NT | 0 | 0 |
| 3 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacterium | E. coli | S. enterica | P. aeruginosa | ||||||
| Standard drug | 13 | 8 | 15 | ||||||
| Concentration (mg/mL) | 10 | 5 | 2.5-0.04 | 10 | 5 | 2.5-0.04 | 10 | 5 | 2.5-0.04 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 |
| 2 | NT | 10 | 0 | NT | 0 | 0 | NT | 10 | 0 |
| 3 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 |
Table II: Antibacterial activity of the compounds by MIC and MBC tests
| Minimum inhibitory concentrations (mg/mL) | Minimum bactericidal concentrations (mg/mL) | |||||||
| Bacteria | (1) | (2) | (3) | The order of activity | (1) | (2) | (3) | The order of activity |
| S. aureus | 2.5 | 1.2 | 2.5 | 1=3<2 | 5 | 5 | 10 | 3<1=2 |
| E. faecalis | 2.5 | 2.5 | 2.5 | 1=2=3 | 10 | >10 | >10 | 3, 2<1 |
| B. subtilis | 1.2 | 1.2 | 0.6 | 1=2<3 | 5 | 5 | 2.5 | 1=2<3 |
| E. coli | 2.5 | 1.2 | 2.5 | 1=3<2 | 10 | 5 | 10 | 1=3<2 |
| S. enterica | 5 | 2.5 | 5 | 1=3<2 | 5 | 2.5 | >10 | 3<1<2 |
| P. aeruginosa | 2.5 | 1.2 | 2.5 | 1=3<2 | 10 | 5 | 10 | 1=3<2 |
Result and Discussion
The antibacterial activity and inhibition zone around tested compounds can be attributed to their bactericidal effect that is due to killing of bacteria or their bacteriostatic effect that inhibits bacterial growth and colony formation through blocking of active sites on surface or inside bacterial cell (Sedaghat et al., 2013). The MIC was defined as the lowest concentration of a substance that inhibited visible growth of the microorganisms. The MBC was the lowest concentration at which no colony formulation was observed on the agar plates, determined by spreading 100 mL of each microorganism on a MHA plate. These plates were incubated at 37°C for 24 hours. The range of antimicrobial activity more than 30 mm shows potent activity, for 21-30 mm inhibition activity is strong activity, 16-20 mm is moderate activity, 10-15 mm is weak activity and below 10 mm is little or no activity (Jeon et al., 2014) or Inhibition zone more than 12 mm shows significant activity, for 10-12 mm inhibition activity is good, 7-9 mm is low, and below 7 mm is non-significant activity (Ahmad et al., 2008).
All the compounds were tested against Gram-positive and Gram-negative bacteria. The structure activity relationship analyses of the compounds were compared. The compounds were screened for Staphylococcus aureus and compounds (2) and (3) had lower activities than the standard tetracycline, and compound (1) exhibited no activity. The compounds (1-3) were screened for Enterococcus faecalis, and had no activity. The compounds were screened for Bacillus subtilis and compound (1) had low activity compared with the standard tetracycline and compounds (2) and (3) exhibited no activity. The compounds (1-3) were screened for Escherichia coli and compounds (1) and (3) exhibited no activity, while compound (2) was found to have low activity compared with the standard tetracycline. The compounds (1-3) were screened for Salmonella enterica, the compound (3) had an equipotent activity compared with the standard tetracycline, while compounds (1) and (2) had no activity. The compounds were screened for Pseudomonas aeruginosa, whereby the compound (1) was less active compared with the standard tetracycline, while the other compounds (2) and (3) had no activity.
The PhPzO (10 mg/mL) and PrPzO (5 mg/mL) exhibited the highest activity against Staphylococcus aureus with zone of inhibition 13 mm. The bacterial zones of inhibition values (mm) of the test compunds are given in Table I.
Table I shows that S. aureus strain is more susceptible to the inhibition growth effect of the compounds. MePzO was found to show maximum activity against B. subtilis and P. aeruginosa at 10 mg/mL with zone of inhibition 12 and 8 mm, respectively. S. aureus and S. enterica showed maximum activity by PhPzO with zone of inhibition 13 and 8 mm, respectively.
Minimum inhibitory concentrations (MICs) are defined as the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation, and minimum bactericidal concentrations (MBCs) as the lowest concentration of an antimicrobial that will prevent the growth of an organism after subculture on the antibiotic-free media (Andrews, 2001). The MIC is a measure of the potency of an antimicrobial compounds. The highest MIC and MBC values of microorganisms are clear indications that compounds are less effective on some bacteria, while the low MIC and MBC values for bacteria are indications of the efficacy of the compounds. Results of MIC and MBC are shown in Table II.
The results show that the PhPzO, PrPzO and MePzO exhibit significantly enhanced activity toward all bacteria under study with MIC at range 0.625-5 mg/mL, 1.25-2.5 mg/mL and 1.25-5 mg/mL, respectively. The PrPzO has the highest activity against E. coli, P. aeruginosa, S. aureus and B. subtilis at 1.25 mg/mL and is least effective against S. enterica and E. faecalis at 2.5 mg/mL. Salmonella enterica is the least affected organism by PhPzO and MePzO at MIC 5 mg/mL; and Bacillus subtilis is most affected at MIC 0.625 mg/mL and 1.25 mg/mL, respectively.
The MBC of MePzO for S. aureus, B. subtilis, and S. enterica was 5 mg/mL and 10 mg/mL for E. faecalis, E. coli and P. aeruginosa.
The MBC of PrPzO and PhPzO for tested bacteria were in the range of 2.5 to >10 mg/mL. The result MBC of PrPzO and PhPzO showed the lowest MBC of 2.5 mg/mL were shown by S. enterica and B. subtilis. The order of activity for the studied compounds was shown in Table II.
Conclusion
The MIC and MBC values of the compounds indicated that compound (2) has the highest activity against Gram-negative bacteria.
References
Abood NA, Al-Shlhai RA. Theoretical study of molecular structure, IR and NMR spectra of pyrazolone and its derivatives. J Chem Pharmaceut Res. 2012; 4: 1772-81.
Ahmad MS, Hussain M, Hanif M, Ali S, Qayyum M, Mirza B. Di- and triorganotin (IV) esters of 3,4-methylenedioxyphe-nylpropenoic acid: Synthesis, spectroscopic characterization and biological screening for antimicrobial, cytotoxic and antitumor activities. Chem Biol Drug Des. 2008; 71: 568-76.
Al-Haiza MA, El-Assiery SA, Sayed GH. Synthesis and potential antimicrobial activity of some new compounds containing the pyrazol-3-one moiety. Acta Pharm. 2001; 51: 251–61.
Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001; 48: 5-16.
Brogden RN. Pyrazolone derivatives. Drugs 1986; 32: 60–70.
Chao EY, Caravella JA, Watson MA, Campobasso N, Ghisletti S, Billin AN. Structure guided design of N-phenyl tertiary amines as transrepression selective liver X receptror modulators with anti-inflammatory activity. J Med Chem. 2008; 51: 5758–65.
Chohan ZH, Scozzafava A, Supuran CT. Synthesis of biologically active Co(II), Cu(II), Ni(II) and Zn(II) complexes of symmetrically 1,1′â€disubstituted ferroceneâ€derived compounds. Synth React Inorg Met.-Org Chem. 2003; 33: 241-57.
El-Hawash SAM, Badawey ESAM, El-Ashmawey IM. Nonsteroidal antiinflammatory agents-part-2 anti-inflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin-5-ones and 1,2,4,5,6,7-3H-hexahydroindazol-3-ones. Eur J Med Chem. 2006; 41: 155–65.
Gursoy A, Demirayak S, Capan G, Erol K, Vural K. Synthesis and preliminary evaluation of new 5-pyrazolinone derivatives as analgesic agents. Eur J Med Chem. 2000; 35: 359–64.
Hadi V, Koh YH, Sanchez TW, Barrios D, Neamati N, Jung KW. Development of the next generation of HIV-1 integrase inhibitors: Pyrazolone as a novel inhibitor scaffold. Bioorg Med Chem Lett. 2010; 20: 6854–57.
Higashi Y, Jitsuikia DD, Chayamab K, Yoshizumia M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Pat Cardiovasc Drug Discovery. 2006; 1: 85–93.
Himly M, Jahn-Schmid B, Pittertschatscher K, Bohle B, Grub-mayr K, Ferreira F, Ebner H, Ebner C. Ig E-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J Allergy Clin Immunol. 2003; 111: 882–88.
Jeon JH, Park JH, Lee HS. 2-isopropyl-5-methylphenol isolated from Ruta graveolens and its structural analogs show antibacterial activity against food-borne bacteria. J Korean Soc Appl Biol Chem. 2014; 57: 485-90.
Khalil AK, Hassan MA, Mohamed MM, El-Sayed AM. Metal salt-catalyzed diazocoupling of 3-substituted-1H-pyrazol-2-in-5-ones in aqueous medium. Dyes Pigments. 2005; 66: 241-45.
Kikuchi K, Kawahara K, Miyagi N, Uchikado H, Kuramoto T, Morimoto Y, Tancharoen S, Miura N, Takenouchi K, Oyama Y, Shrestha B, Matsuda F, Yoshida Y, Arimura S, Mera K, Tada K, Yoshinaga N, Maenosono R, Ohno Y, Hashiguchi T, Maruyama I, Shigemori M. Edaravone: A new therapeutic approach for the treatment of acute stroke. Med Hypotheses. 2010; 75: 583–85.
Kim KR, Kwon JL, Kim JS, No Z, Kim HR, Cheon HG. EK-6136 (3-methyl-4-(O-methyl-oximino)-1-phenylpyrazolin-5-one): A novel Cdc25B inhibitor with antiproliferative activity. Eur J Pharmacol. 2005; 528: 37–42.
Makhija MT, Kasliwal RT, Kulkarni V M, Neamati N. De novo design and synthesis of HIV-1 integrase inhibitors. Bioorg Med Chem. 2004; 12: 2317–33.
Manojkumar P, Ravi TK, Gopalakrishnan S. Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazo-nopyrazolones containing coumarin moiety. Eur J Med Chem. 2009; 44: 4690–94.
Perrin DD, Armarego WLF. Purification of laboratory chemicals. 3rd ed. Oxford, Pergamon, 1988.
Ramana Kumar K, Raghavendra Guru Prasad A, Srilalitha V, Narayana Swamy G, Ravindranath LK. Synthesis and electrochemical investigations on certain pyrazolin-5-ones. Scientia Iranica C. 2012; 19: 605–18.
Raman N, Kulandaisamy A, Jeyasubramanian K. Synthesis, structural characterization, redox, and antibacterial studies of 12- membered tetraaza macrocyclic Cu(II), Ni(II), Co(II), Zn(II), and VO(IV) complexes derived from 1,2-(diimino-40-antipyrinyl)-1,2-diphenylethane and o-phenylenediamine. Synth React Inorg Met. 2004; 34: 17–43.
Ramaraj S, Ramachandran V Pradeepchandran, Jauaveera KN, Vijainad R, Palani KA. Computational approach of benzimidazole containing pyrazolin-5-one derivatives as targeted antifungal activity. Int J Health Nutr. 2010; 1: 1–6.
Ratnadeep SJ, Priyanka GM, Santosh DD, Sanjay KD, Charansingh HG. Synthesis, analgesic and anti-inflammatory activities of some novel pyrazolins derivatives. Bioorg Med Chem Lett. 2010; 20: 3721–25.
Sedaghat T, Golalzadeh A, Motamedi H. Diorganotin complexes with N(4)-phenylthiosemicarbazones: Synthesis, spectroscopic characterization, and antibacterial activity. Phosphorus Sulfur Silicon. 2013; 188: 1694-702.
Song LP, Zhu SZ. Regioselective synthesis of fluorinated pyrazole derivatives from trifluoromethyl-1,3-diketone. J Fluorine Chem. 2001; 111: 201-05.
Tsujita K, Shimomura H, Kawano H. Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. Am J Cardiol. 2004; 94: 481–84.
Vincent TA. Current and future antifungal therapy: New targets for antifungal agents. J Antimicrob Chemother. 1999; 44: 151–62.
Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994; 268: 1597–604.
Zhang P, Li W, Li L, Wang N, Li X, Gao M, Zheng J, Lei S, Chen X, Lu H, Liu Y. Treatment with edaravone attenuates ischemic brain injury and inhibits neurogenesis in the subventricular zone of adult rats after focal cerebral ischemia and reperfusion injury. Neuroscience 2012; 201: 297–306.