Quercetin potentiates the effect of gamma-delta T cells via modulating the expressions of Granzyme B, perforin and IFN-gamma and also regulates the Wnt/beta-catenin signaling pathway in human colon cancer cells
Abstract
Cancer accounts as one of the leading causes of morbidity and mortality. Recent studies focus on the efficiency of phytochemicals in cancer therapy. Influence of quercetin, a flavonoid on the effect of gamma-delta T cells and Wnt/beta-catenin signalling pathway in human colon cancer cells (HT55 and HCT116) was investigated. Quercetin at 15-120 µM was observed to markedly reduce the viability of HT55 and HCT116 cells. Quercetin exposure significantly increased gamma-delta T cell proliferation and also raised the expressions of granzyme B (Gra B), perforin (PFP), and interferon-gamma (IFN-gamma) in gamma-delta T cells. Reduced beta-catenin expression with increased expressions of phosphorylated- beta-catenin, axin1 and 2 were observed in HT55 and HCT116 cells on exposure to quercetin. However beta-actin expression was found to be not much altered. The results suggest that quercetin was able to efficiently potentiate the effect of gamma-delta T cells and modulate Wnt/beta-catenin signaling pathway.
Introduction
Colon cancer has multiple transition steps due to the accrual of genetic errors in genes involved in apoptosis and cell proliferation (Davies et al., 2005; Watson, 2006). The most frequent cause is the dysregulation of Wnt/beta-catenin signalling pathway that plays a vital role in normal cellular responses and in tumorigenesis (Logan and Nusse, 2004; Clevers, 2006). Genetic defects that lead to aberrant activation of Wnt/beta-catenin signalling are reported in over 90% of sporadic colon cancer cases (Miyaki et al., 1994; Klaus and Birchmeier, 2008). beta-Catenin is a key effector that determines the activity of Wnt/beta-catenin signalling. Upon activation of Wnt signal, beta-catenin translocates into the nuclear region and forms a ternary complex with transcription factors - T-cell factor/ lymphoid enhancer factor to activate genes involved in cell proliferation (Reya and Clevers, 2005; Espada et al., 2009).
The crucial role of gamma-delta T cells in immune regulation, antitumor immunosurveillance and primary immune response has been recognized (Born et al., 2006). gamma-delta T cells have been reported to exhibit potent MHC unrestricted lytic activity against various tumor cells in vitro, which strongly suggests that gamma-delta T cells could be used in anticancer immunotherapy. Previous observations have recently aided the development of novel immunotherapeutic approaches aimed at gamma-delta T cell activation (Zhu et al., 2013).
Studies have reported that consumption of fruits and vegetable may decrease the risk of cancer and immunodysfunctions (Block et al., 1992; Joshipura et al., 2001). Much of the health effects of fruits and vegetables have been attributed to phytochemicals as flavonoids (Ames et al., 1993; Boyer et al., 2004).
Quercetin, a flavonoid, is abundantly present in various fruits, vegetables, seeds, nuts, tea and red wine. The antiproliferative efficacy of quercetin has been reported previously (Kang and Liang, 1997; Boyer et al., 2004; Suh et al., 2010). The influence of quercetin on gamma-delta T cells and on the Wnt/beta-catenin signalling pathway in human colon cancer cells was investigated in the study.
Materials and Methods
Antibodies and reagents: Human Colon Cancer cell lines - HT55 and HCT116 were obtained from Sigma-Aldrich, St. Louis, MO, USA. Monoclonal antibody fluorescein isothiocyanate (FITC)-conjugated anti-TCR gamma-delta, phycoerythrin (PE)-conjugated-anti-Granzyme B (GraB), PE-conjugated anti-perforin (PFP), APC conjugated anti-IFN-gamma and control IgGs, mouse anti-beta catenin, mouse anti-beta-actin were purchased from BD Biosciences. Rabbit anti-p-beta-catenin (S45) was from Cell Signaling Technology, Danvers, MA, USA. Rabbit- anti-axin1, anti-axin2 and recombinant human interleukin-2 (rhIL-2) were purchased from Sigma-Aldrich, St. Louis, MO, USA. All other chemicals used in the study were purchased from Sigma-Aldrich, USA unless otherwise mentioned.
Cell viability assay: The cell viability assay was performed as previously described (Pan et al., 2010; Zheng et al., 2010). The human colon cancer cells were seeded in 96-well microplates (2 x 105 cells/well). After the cells reached 70% confluence, the cells were treated with quercetin (15, 30, 60 and 120 µM) and 50 µM, 5-fluorouracil (5-Fu) as positive control for 72 hours. The final concentration of DMSO in culture medium was maintained at 0.05% (Jaramillo et al., 2010; Pan et al., 2010). After 72 hours of incubation, 10 µL of MTT (5 mg/mL) in PBS was added to each well at a final concentration of 0.5 mg/mL and incubated for 4 hours. The supernatant was discarded and 100 µL of a solution containing 10% SDS (pH 4.8), HCl (0.01 M) and 5% isobutyl alcohol was added to each well and mixed thoroughly to dissolve the formazan crystals. Cell viability was measured by reading the absorbance at 570 nm using an enzyme-linked immunosorbent assay (ELISA) reader (RT6000, Guangdong, China). Viability was expressed as a percentage of absorbance values in treated cells to that in control cells:
cell viability (%) = ODtest/ ODcontrol x 100%
Expression of GraB, PFP and IFN-gamma: Purified human peripheral blood mononuclear cells (PBMCs) were incubated in RPMI1640 medium supplemented with 100 mL/L fetal calf serum, 50 mL/L human AB serum, 2 ug/L isopentenyl pyrophosphate (IPP) and 100 IU/mL rhIL-2, for 10 days at 37°C with 5% CO2. At the end of incubation period, the cells were harvested and purified populations of the gamma-delta T cells were obtained by staining with anti-TCRgamma-delta-FITC and goat anti mouse-IgG1, κ FITC that served as isotype control. The stained cells were analyzed by flow cytometry. Cell viability was determined using trypan blue exclusion.
Purified gamma-delta T cells were plated in 6-well plates at a density of 1x106 cells/mL (3 mL/well), incubated in a humidified 5% CO2 incubator at 37°C for 24 hours, and then incubated in the presence of quercetin at concentrations of 15-120 µM at 37°C, 5% CO2 for 48 hours. The cells were collected by centrifuging at 2000 rpm for 5 min, washed with PBS, and the concentration was adjusted to 1x1010 cells/mL for subsequent immunofluorescent staining. Twenty microliters of anti-TCR-gamma-delta FITC was added to each well, where gamma-delta T cells in 50 uL of medium contained 0.1% azide. They were mixed thoroughly and incubated for 30 min in the dark at 4°C. One hundred microliters fixation buffer was added to each well followed by incubation in dark at 4°C for 15 min, washed with PBS twice, and centrifuged for 5 min at 1500 rpm. The supernatants were discarded. The cells were permeabilized with 100 uL 0.5% saponin and stained either with PE-anti-PFP antibody or PE-anti-GraB antibody, or their PE-IgG istotype control antibody. The cells were incubated in dark at 4°C for 15 min followed by addition of 3 mL PBS and centrifuged at 2000 rpm for 5 min. The cells were resuspended in 0.5 mL of PBS and finally analyzed for GraB and PFP by flow cytometer.
The cultured gamma-delta T cells after incubation with quercetin were stained with FITC-anti-TCRgamma-delta antibody followed by fixation and permeabilization for intracellular IFN-gamma staining using APC-anti-IFN-gamma. APC-conjugated mouse IgG1 was used as an isotype control. Gating was performed on gamma-delta T cells and the percentage of IFN-gamma producing cells was calculated.
Western blotting: The human colon cancer cells HT55 and HT116 were incubated with quercetin (30-120 µM) as described in cell viability assay. Total cell lysates followed incubation with quercetin was prepared in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS). Protein concentration was determined and equal amount of protein samples were subjected to 8-13% SDS-PAGE. Separated proteins were transferred to PVDF membranes (Millipore, Bedford, MA, USA). After blocking for 1 hour at room temperature in 5% bovine serum albumin in PBS-Tween 20, membranes were probed overnight at 4oC with the following antibodies: mouse anti-beta catenin, mouse anti-beta-actin, rabbit anti-p-beta-catenin (S45), anti-axin1, anti-axin2. The membranes were incubated with corresponding secondary anti-bodies for 2 hours at room temperature, and later visualized with an enhanced chemiluminescence (ECL) detection kit (Lab Frontier, Suwon, Korea). The blots were analyzed by LAS4000 (Fuji Film Corp., Tokyo, Japan). Band intensity was measured using ImageJ software (National Institute of Health, USA).
LDH release assay: Purified gamma-delta T cells cultured for 10 days were plated in 6-well plates in quintuplicate at a density of 1x106 cells/mL (3 mL/well), placed in a humidified 5% CO2 incubator at 37°C for 24 hours were incubated with quercetin (15-120 µM) at 37°C, 5% CO2 for 48 hours. The cells incubated in the absence of quercetin served as the control group. gamma-delta T cells that have been pretreated or not with quercetin were resuspended at the final concentration of 2x109 cells/mL, and 100 uL was then added to round-bottom polystyrene tubes together with human colon cancer cells (100 uL). The cells were incubated for 6 hours at 37°C, in 5% CO2, and then centrifuged at 1500 rpm for 10 min. Supernatants were collected.
LDH leakage was assessed with an LDH assay kit (Jiancheng BioEngineering, Nanjing, China). Briefly, 20 µl of culture supernatants after different treatments and incubation were used for the leakage analysis according to the manufacturer instructions as described previously (Yu et al., 2009). The absorbance was read at 450 nm and LDH activity was expressed as units per liter (U/L). LDH activity (U/L) = (ODtest - ODcontrol)/(ODstandard - ODblank) x Cstandard x Ndilution factor x 1000.
Statistical analysis: Data are expressed as mean ± SD from at least three independent experiments. One-way analysis of variance (ANOVA) at p<0.05 was considered statistically significant. The statistical analyses were performed using SPSS 17.0 software.
Results
The effects of quercetin at different concentrations (15-120 µM) on gamma-delta T cells and on colon cancer cells (HT55 and HCT116) were assessed. In cancer immunotherapy, repetitive stimulation of gamma-delta T cells is crucial for comparatively extended series of treatments. Thus in this study the effect of quercetin on the reactivity of gamma-delta T cells was evaluated. Quercetin induced a dose dependent proliferation of gamma-delta T cells after 48 hours of exposure. Cell proliferation increased from 16.7% in control culture (not exposed to quercetin) to 46.8% at 30 µM. Quercetin at a concentration of 120 µM resulted in significant (p<0.05) raise in cell proliferation to 61.8% (Figure 1).
Figure 1: Effect of quercetin on the proliferation of gamma-delta T cells. Values are represented as mean ± SD; n=3; arepresents p<0.05 compared with control as determined by one-way-ANOVA
The growth modulating effects of quercetin in colonic cancer cells was evaluated by MTT assay. The proliferation of cancer cells was significantly (p<0.05) inhibited in dose dependent manner upon incubation with quercetin for 72 hours (Figure 2). Exposure to 15 µM quercetin evidenced a decline in cell viability though not significant as compared to control cells that were not exposed to quercetin. The cell viability was observed to be 82.1% in HCT116 cells and 83.1% HT55 on exposure to 15 µM quercetin. The decrease in cell viability was found to be nearly 2 folds in HT55 and HCT116 cells on incubation with 60 µM as against control. Sharp decline in cell viability was observed in cancer cells that were exposed to 5-FU. Incubation with 120 µM quercetin resulted in a significant drop in viability percentage closer to standard anti-cancer agent 5-FU.
Figure 2: Effect of quercetin on the viability of colon cancer cells. Values are represented as mean ± SD; n=3; arepresents p<0.05 compared with control as determined by one way-ANOVA
Exposure to quercetin for 48 hours was observed to significantly (p<0.05) increase the expression of GraB and PFP in a dose dependent manner as compared with the control group, gamma-delta T cells that were not cultured with various concentrations of quercetin (Figure 3).
Figure 3: Effect of quercetin on GraB and PFP expression of gamma-delta T cells. Values are represented as mean ± SD; n=3; arepresents p<0.05 compared with control as determined by one way-ANOVA
The percentage of PFP positive gamma-delta T cells increased from 39.5% in the control cells to 70.1% in the cells exposed to quercetin at 120 µM. Exposure to quercetin at 60 µM resulted in 2 fold increase in PFP expression.
The expression of GraB in the gamma-delta T cells on incubation with various concentrations of quercetin evidenced marked raise in the levels of GraB expression. The concentration of 120 µM was observed to produce 81.2% raise as compared to control groups. In the cells exposed to 15 µM, raise in GraB expression was observed to be insignificant. Higher concentrations above 30 µM displayed higher expression of the GraB than 15 µM group. The percentage of GraB positive gamma-delta T cells increased from 50.8% in the control cells to 75.9% in cells treated with 60 µM quercetin (Figure 3).
The percentage of IFN-gamma producing gamma-delta T cells was found to be highest at the concentration of 60 µM quercetin whereas as at 120 µM quercetin concentration, the expression of IFN-gamma was slightly lesser. However, significant raise (p<0.05) in the percentage of IFN-gamma producing gamma-delta T cells was presented on incubation with 30 µM and higher concentrations as compared against control cancer cells. IFN-gamma producing gamma-delta T cells increa sed from 47.1% at 15 µM to 70.1% at 60 µM (Figure 4).
Figure 4: Effect of quercetin on IFNgamma expression of gamma-delta T cells. Values are represented as mean ± SD; n=3; arepresents p<0.05 compared with control as determined by one way-ANOVA
The effect of quercetin at various concentrations on the cytotoxicity of gamma-delta T cells against colon cancer cells were analyzed by LDH release assay. Incubation with quercetin resulted in a significant raise in the cytotoxic potency of gamma-delta T cells which increased with increasing concentration of quercetin from 15 uM to 120 uM (Figure 5). Quercetin at 15 uM resulted in increase in the release of LDH in HT55 and HCT116 cells. This indicates marked (p<0.05) increase in the cytotoxic activity of gamma-delta T cells when exposed to 30 uM quercetin. The LDH activity raised 2-folds in the media suggestive of loss of membrane integrity. gamma-delta T cells incubated with 120 uM quercetin showed the highest cytotoxic activity as evidenced by the marked increase in the LDH release to the culture media.
Figure 5: Influence of quercetin on the effect of gamma-delta T cells on HT55 and HCT116 cells. Values are represented as mean ± SD; n=3; *represents p<0.05 compared with control as determined by one way-ANOVA
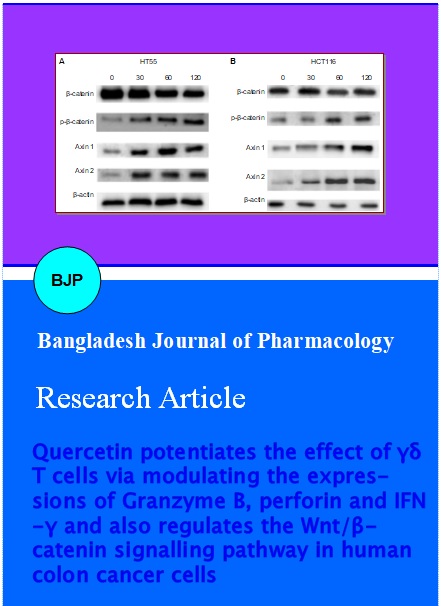
Since beta-catenin is a key effector that determines the activity of Wnt/beta-catenin signalling pathway and also as phosphorylation regulates beta-catenin degradation, the expression of beta-catenin and phosphorylated beta-catenin was assessed. Western blot analysis revealed increase in the phosphorylated forms of beta-catenin in contrast to total beta-catenin levels in a dose-dependent manner. This demonstrated that quercetin increased phosphorylation of beta-catenin and ensuing degradation in the colon cancer cells (Figure 6).
Figure 6: Western blot analysis of Wnt/beta-catenin signalling pathway proteins (A) HT55 cells. (B) HCT116 cells. Lane 1- 4: Concentration of quercetin in µM
The expression of axin1 and axin2 were assessed in HT55 and HCT116 cells. These results revealed that quercetin up-regulated axin levels. The up-regulation was the highest with 120 µM quercetin (Figure 6). Exposure to various concentrations of quercetin did not induce any significant change in the expressions of beta-actin in both HT55 and HCT116 cells as compared to control cells that were not exposed to quercetin. Though quercetin at 60 and 120 µM slightly raised the expression levels of actin, marked increase was not observed.
Discussion
gamma-delta T cells are widely distributed in the various tissues of the body. The cells have antigen recognition properties and effect the regulation of immune response through cell contact dependent mechanism and secretion of cytokines (Uchida et al., 2007). Previous studies have reported that gamma-delta Τ cells cultured from human peripheral blood may be vital in anticancer surveillance (Campillo et al., 2007).
In the present study, incubation with quercetin (15-120 uM) induced a dose dependent proliferation to gamma-delta T cells after 48 hours of exposure. Higher (120 uM) concentration of quercetin resulted in a multi-fold raise in the proliferation of gamma-delta T cells. The results suggest that proper concentrations of quercetin could promote proliferation of gamma-delta T cells that can augment immune responses against cancer cells.
PFP plays a crucial role in cell apoptosis mediated by T lymphocyte (Hayashida et al., 2000); nevertheless, it is granzyme which is the actual killer of target cells (Konno et al., 1999). Perforin\granzyme-induced apoptosis is the main pathway through which the cytotoxic lymphocytes mediate antitumor immunity. Once the T cells kill the target cells, the PFP bores a hole in the target cell wall following which Granzyme degrades DNA inducing apoptosis of target cells (Berke, 1997). Granzyme B is the most important granzyme in apoptosis and it can induce target cell apoptosis through caspase activation, direct cleavage of specific intracellular substrates, and induction of mitochondria damage as well (Heibein et al., 1999; Barry and Bleackley, 2002; Lieberman, 2003; Koning et al., 2009). The results of the present investigation showed that the expression of granzyme B and PFP on Îgamma-delta T cells incubated with quercetin increased markedly in a dose dependent way in line with significant raise in gamma-delta T cells thus suggesting that quercetin can enhance anti-tumor effect through immunoregulation.
Previous studies have demonstrated different pathways through which gamma-delta T cells may exert anticancer effects including direct killing of transformed cells and early IFN-gamma production. Incubation of gamma-delta T cells with quercetin evidenced significant elevation in IFN-gamma expression that was observed to increase with concentration of quercetin. It could be suggested that quercetin also enhances the activity of gamma-delta T cells by increasing IFN-gamma production.
In addition, the cytotoxicity of gamma-delta T cells pretreated with different concentrations of quercetin for 48 hours on colon cancer cells (HT55 and HCT116) was analyzed by LDH release assay. The results indicated that quercetin could enhance the cytotoxic activity of gamma-delta T cells simultaneously with the gradual increase in concentration. Thus, quercetin efficiently increases the potency of gamma-delta T cells on human colon cancer cells through increasing gamma-delta T cell proliferation and by up-regulating the expression of Granzyme B, PFP and IFNgamma.
Improper activation of Wnt\beta-catenin signalling path-way has been demonstrated as the best characterized mechanism of carcinogenesis in colon cancer. It is well recognized that the constitutive Wnt signaling is essential for the survival of colon cancer cells, and repression of the Wnt signaling pathway could result in cancer cell growth inhibition (Verma et al., 2003). The effect of quercetin on the Wnt\beta-catenin signalling pathway components was studied. Wnt signaling pathway is a vital pathway involved in regulating cell proliferation, differentiation and morphogenesis in different organs (Reya and Clevers, 2005). In colon cancer, the functional APC complex involved in beta-catenin degradation is disrupted due to mutations in APC or beta-catenin (Barker and Clevers, 2006; Klaus and Birchmeier, 2008) that results in nuclear accumulation of beta-catenin, leading to transcription of various genes associated with cell proliferation and survival. Thus, the identification of novel inhibitors of Wnt\beta-catenin signaling has received much attention as a potential new means to regulate and prevent colon cancer.
Quercetin (15-120 µM) reduced the cell viability of the colon cancer cells, HT55 and HCT116. The cell viability reduced with a gradual increase in concentration of quercetin. The results of the western blotting analysis revealed that incubation with various concentrations of quercetin modulated the expressions of beta-catenin and phosphorylated forms of beta-catenin in the HT55 and HCT116 cells. The expression of beta-catenin decreased with elevated expressions of phosphorylated forms of beta-catenin. Decrease of beta-catenin expression would decrease transcription of cell survival and cell proliferation related genes. The expression of axin1 and axin2 were also observed to be elevated. These findings suggested that quercetin may possibly up-regulate phosphorylation of beta-catenin by increasing axin levels and thus regulate the Wnt\beta-catenin signalling path-way.
The results of the study were in line with previous studies of other flavonids like fisetin. Fisetin was reported as an inhibitor of Wnt\beta-catenin signalling (Syed et al., 2011). Apigenin (40 uM) reduced the levels of beta-catenin and Dsh proteins and accelerated the degradation of beta-catenin in the first two hours of treatment promoting cell cycle arrest in breast cancer cells (Song et al., 2000; Landesman-Bollag et al., 2001). EGCG (epigallocatechin gallate) was found to inhibit Wnt signalling in a dose dependent manner in, lung cancer, breast cancer, colon cancer and in normal cells where Wnt signaling was hyperactivated (Dashwood et al., 2002; Kim et al., 2006; Mount et al., 2006; Pahlke et al., 2006; Gao et al., 2009).
Conclusion
Quercetin could effectively aid in immunotherapy of cancer through augmenting gamma-delta T cells and by regulating Wnt\beta-catenin signalling pathway.
References
Ames B, Shigenaga M, Hagen T. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993; 90: 7915-22.
Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006; 5: 997-1014.
Barry M, Bleackley RC. Cytotoxic T lymphocytes: All roads lead to death. Nat Rev Immunol. 2002; 2: 401-09.
Berke G. Killing mechanisms of cytotoxic lymphocytes. Curr Opin Hematol. 1997; 4: 32-40.
Bjeldanes JF, Chang GF. Mutagenic activity of quercetin and related compounds. Science 1997; 197: 577-78.
Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992; 18: 1-29.
Born WK, Reardon CL, O'Brien RL. The function of gamma delta T cells in innate immunity. Curr Opin Immunol. 2006; 18: 31-38.
Boyer J, Brown D, Liu RH. Uptake of quercetin and quercetin 3-glucoside from whole onion and apple peel extracts by Caco-2 Cell monolayers. J Agric Food Chem. 2004; 52: 7172-79.
Campillo JA, Martinez-Escribano JA, Minguela A, Lopez-Alvarez R, Marin LA, Garcia-Alonso AM, Bensussan A, Alvarez-Lopez MR. Increased number of cytotoxic CD3+ CD28− gamma delta T cells in peripheral blood of patients with cutaneous malignant melanoma. Dermatology 2007; 214: 283-88.
Clevers H. Wnt/β-catenin signaling in development and disease. Cell 2006; 127: 469-80.
Dashwood WM, Orner GA, Dashwood RH. Inhibition of β-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): Minor contribution of H(2)O(2) at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002; 296: 584-88.
Davies RJ, Miller R, Coleman N. Colorectal cancer screening: prospects for molecular stool analysis. Nat Rev Cancer. 2005; 5: 199-209.
Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009; 20: 743-52.
Duthie SJ, Dobson VL. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr. 1999; 38: 28-34.
Espada J, Calvo MB, Diaz-Prado S, Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol. 2009; 11: 411-27.
Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong M, Zhi X, Jablon DM, You L. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res. 2009; 29: 2025-30.
Gerritsen ME. Flavonoids: Inhibitors of cytokine induced gene expression. Adv Exp Med Biol. 1998; 439: 183-90.
Hayashida M, Kawano H, Nakano T, Shiraki K, Suzuki A. Cell death induction by CTL: Perforin/granzyme B system dominantly acts for cell death induction in human hepatocellular carcinoma cells. Proc Soc Exp Biol Med. 2000; 225: 143-50.
Heibein JA, Barry M, Motyka B, Bleackley RC. Granzyme B-induced loss of mitochondrial inner membrane potential (Delta Psi m) and cytochrome c release are caspase independent. J Immunol. 1999; 163: 4683-93.
Jaramillo S, Lopez S, Varela LM, Rodriguez-Arcos R, Jimenez A, Abia R, Guillen RG, Muriana FJ. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J Agric Food Chem. 2010; 58: 10869-75.
Joshipura K, Hu F, Manson J, Stampfer M, Rimm E, Speizer F, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett W. The effect of fruit and vegetable intake on risk of coronary heart disease. Ann Intern Med. 2001; 134: 1106-14.
Kang TB, Liang MC. Studies on the inhibitory effects of quercetin on the growth of HL460 leukemia cells. Biochem Pharmacol. 1997; 54: 1013-18.
Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells: Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006; 281: 10865-75.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008; 8: 387-98.
Koning PJ, Kummer JA, Bovenschen N. Biology of granzyme M: A serine protease with unique features. Crit Rev Immunol. 2009; 29: 307-15.
Konno R, Igarashi T, Okamoto S, Sato S, Moriya T, Sasano H, Yajima A. Apoptosis of human endometrium mediated by perforin and granzyme B of NK cells and cytotoxic T lymphocytes. Tohoku J Exp Med. 1999; 187: 149-55.
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001; 227: 153-65.
Lieberman J. The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat Rev Immunol. 2003; 3: 361-70.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol. 2004; 20: 781-810.
Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M, Maeda Y, Iwama T, Mishima Y, Mori T, Koike M. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994; 54: 3011-20.
Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the canonical Wnt signalling path-way regulates deer antler regeneration. Dev Dyn. 2006; 235: 1390-99.
Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006; 54: 7075-82.
Pan MH, Lin CL, Tsai JH, Ho CT, Chen WJ. 3,5,3’,4’,5’-Pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J Agric Food Chem. 2010; 58: 226-34.
Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003; 23: 519-34.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 2005; 434: 843-50.
Sies H. Polyphenols and health: update and perspectives. Arch Biochem Biophys. 2010; 501: 2-5.
Song DH, Sussman DJ, Seldin DC. Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J Biol Chem. 2000; 275: 23790-97.
Suh DK, Lee EJ, Kim HC, Kim JH. Induction of G1/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch Pharm Res. 2010; 33: 781-85.
Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, Setaluri V, Mukhtar H. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011; 131: 1291-99.
Uchida R, Ashihara E, Sato K, Kimura S, Kuroda J, Takeuchi M, Kawata E, Taniguchi K, Okamoto M, Shimura K, Kiyono Y, Shimazaki C, Taniwaki M, Maekawa T. Gamma delta T cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem Biophys Res Commun. 2007; 354: 613-18.
Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003; 9: 1291-300.
Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol. 2006; 57: 107-121.
Yu CH, Kan SF, Shu CH, Lu TJ, Lucy SH, Wang PA. Inhibitory mechanisms of Agaricus blazei murill on the growth of prostate cancer in vitro and in vivo. J Nutr Biochem. 2009; 20:753-64.
Zheng YQ, Xin YW, Shi XN, Guo YH. Cytotoxicity of monascus pigments and their derivatives to human cancer cells. J Agric Food Chem. 2010; 58: 9523-28.
Zhu SP, Liu G, Wu XT, Chen FX, Liu JQ, Zhou ZH, Zhang JF, Fei SJ. The effect of phloretin on human γδ T cells killing colon cancer SW-1116 cells. Int Immunopharmacol. 2013; 15: 6-14.