GC/MS analysis, free radical scavenging, anticancer and beta-glucuronidase inhibitory activities of Trillium govanianum rhizome
Abstract
The current study aims to investigate the phytochemical constituents, antioxidant, beta-glucuronidase inhibitory and anticancer activities of Trillium govanianum rhizome. Phytochemical screening revealed the presence of steroidal glycosides, saponins, sterols, flavonoids and carbohydrates. GC/MS analyses of n-hexane fraction identified 12 compounds, including 70% unsaturated and 30% saturated fatty acids. Higher free radical scavenging capacity was observed in n-hexane and chloroform fraction compared with the other fractions. Based on IC50 (ug/mL) values, antiproliferative activity on HeLa cells was observed for chloroform (0.8 ± 0.2), ethyl acetate (1.4 ± 0.1) and butanol (1.6 ± 0.3) fractions by comparison to anticancer drug doxorubicin (0.3 ± 0.0). Similarly, all fractions exhibited cytotoxicity on PC-3 cells. Moreover, the methanol extract (IC50: 140.8 ± 3.8) and butanol fraction (196.2 ± 1.9) exhibited a moderate level of β-glucuronidase inhibitory activity. These findings may validate the folkloric uses of T. govanianum rhizome in cancer management, and can be a promising candidate as an anticancer agent.
Introduction
Medicinal plants are capitalized in cure of different ailments since time immemorial. This can be attributed to the presence of secondary metabolites, arising from primary metabolism by different biosynthetic path-ways. Important classes of secondary metabolites include alkaloids, glycosides, tannins, steroids, terpenoids, saponins and phenolic compounds. These are biologically active molecules and potential activities of any medicinal plant are primarily due to these metabolites (Okigbo et al., 2009). After isolation of wide varieties of active compounds and exploration of their efficacies and safety profiles, it is proved that medicinal plants have a value in the modern synthetic era. Numerous drugs have entered the international pharmacopoeias through the study of ethnopharmacology and traditional medicine (Patwardhan et al., 2005).
Keeping in view the importance and scope of medicinal plants, the present study was aimed to investigate the phytochemical constituents and biological activities of Trillium govanianum Wall. ex Royle, used in the traditional systems of medicine. T. govanianum belongs to the family Trilliaceae and is mainly distributed in South Asia, from Pakistan to Bhutan, at an altitude of 2700-3800 m (Muhammad, 2011). The genus Trillium includes long lived herbaceous flowering plants and different species are widely distributed throughout the world; the species reported from Pakistan is T. govanianum (Flora of Pakistan). The Asian species of Trillium produce secondary metabolites that are mainly exploit ted in cancer treatments (Huang et al., 2011). North American species of Trillium are known to have antifungal and antibacterial properties (Akihito et al., 2008). In folk medicine, T. govanianum is used to cure dysentery, in wound healing, inflammation, sepsis, menstrual and sexual disorders (Mahmood et al., 2013; Pant et al., 2010; Savita et al., 2013). It has been reported that the powdered plant is used as body and sexual tonic (Khan et al., 2013). Due to the folkloric knowledge of this plant, it is relevant to provide scientific evidence to its traditional uses, as well as to screen this valuable herb for any possible potential biological activity that will provide a base for isolation of targeted lead compounds. In this study, to the best of our knowledge we have explored for the first time, the antioxidant, anticancer and beta-glucuronidase inhibitory activities of T. govanianum rhizome, its phytochemical constituents and proximate fatty acids composition.
Materials and Methods
Plant material
Plants of T. govanianum were collected from Dir Upper, Kohistan Valley (34° 54' and 35° 52' North latitudes and 72° 43' and 73° 57' East longitudes, at an altitude of 2700 -3800 m) in August, 2013. The plant was identified by Mr. Ghulam Jelani (Curator) of the Department of Botany, University of Peshawar and a voucher specimen [No. Bot. 20092 (PUP)] was deposited in the herbarium of the same department.
Extraction and fractionation
The shade dried rhizomes of T. govanianum (7 kg) were grounded and extracted with methanol (40 L) at room temperature for a period of 14 days (3 X 40 L). The combined methanolic extract was evaporated to dryness to yield a brownish gummy residue (512 g), which was further fractionated (solid-liquid partition) into n-hexane (81 g), chloroform (94 g), ethyl acetate ( 85 g) and n-butanol (115 g) fractions.
Phytochemical screening
Different qualitative chemical tests of the n-hexane, chloroform, ethyl acetate, n-butanol and methanol fractions were performed for the determination of plant metabolites like alkaloids, tannins, phenolic compounds, flavonoids, saponins, sterols and carbohydrates, according to the recommended standard protocols (Shome and Joshi, 1984).
Gas chromatography/mass spectrometry (GC/MS)
GC/MS analysis was carried out on a 6890N Agilent gas chromatograph coupled with a JMS 600 H JEOL mass spectrometer. The compound mixture was separated on a fused silica capillary SPBI column, 30 m x 0.32 mm, 0.25 µm film thicknesses, in a temperature program from 50 to 256°C with a rate of 4°C/min with 2 min hold. The injector was at 260°C and the flow rate of the carrier gas (helium) was 1 mL/min. The EI mode of JMS 600 H JEOL mass spectrometer has ionization volt of 70 eV, electron emission of 100 µA, ion source temperature of 250°C and analyzer temperature of 250°C. Sample was injected manually in split mode. Total elution time was 90 min. MS scanning was performed from m/z 85 to m/z 390 (Faizi et al., 2014).
Derivitization
The n-hexane fraction was esterified to the more volatile methyl esters by methanol boron trifluoride method. Hexane solution (5 mL) obtained from the extraction was treated with boron trifluoride methanol (10%) solution and refluxed for 2 min on a water bath, and then 5 mL n-hexane was added, after an another 1 min of reflux, the solution was treated with 15 mL saturated NaCl solution under vigorous stirring. The organic layer was then separated and dried over anhydrous CaCl2 (Botinestean et al., 2012).
GC/MS identification of components
Identification of proximate fatty acid components of the non-polar fraction (n-hexane) was based on the computer evaluation of mass spectra of sample through NIST-based AMDIS (automated mass spectral deconvolution and identification software), direct comparison of peaks and retention times with those for the standard compounds as well as by following the characteristic fragmentation patterns of the mass spectra of particular classes of compounds.
DPPH free radical scavenging assay
The in vitro antioxidant activity was evaluated by DPPH free radical scavenging assay according to method described elsewhere (Lue et al., 2010) with slight modifications. Two milliliters of 0.1 mM DPPH free radical solution in methanol were added to 1 mL of different concentrations (1, 10, 30, 50, 100, 200 μg/mL) of the fractions or standards in methanol. The solutions were shaken thoroughly on a vortex (Gyromixer, Pakland Scientific Production, Pakistan) and incubated in the dark at ambient temperature for 30 min. Absorbance was then measured at 517 nm using UV/Visible spectrophotometer (Lambda 25, PerkinElmer, USA) against control which consisted of 0.1 mM DPPH free radical solution without extracts or standards. Blank consisted of methanol alone. Ascorbic acid and BHT were used as standards. The percent DPPH free radical scavenging was calculated using the following formula: DPPH (%) = [(AI-AII/A1) x 100], where AI is the absorbance of the control reaction and AII is the absorbance in the presence of the sample.
Anticancer assay
The cytotoxic activity of samples was determined by the MTT assay, according to Baydoun and Mosmann with slight modification (Baydoun et al., 2013; Mosmann, 1983), on two cancer cell lines, i.e. HeLa (cervical cancer cells) and PC-3 (prostate cancer cells). For the assay, cells were grown in DMEM (Dulbecco's modified Eagle medium) and MEM (modified Eagle's medium) containing 10% FBS and 2% antibiotic (penicillin and streptomycin) and maintained at 37°C, in 5% CO2, for 24 hours, in a flask. Cells were plated (1 x 105 cell/mL) in 96-well flat bottom plates and incubated for 24 hours for cell attachment. Various concentrations of test sample/fractions ranging from 1.25-20 µM were added into the well and incubated for 48 h. A 50 µL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 0.5 mg/mL] aliquot was added to each well 4 hours before the end of incubation. Medium and reagents were aspirated and 100 µL DMSO was added and mixed thoroughly for 15 min to dissolve the formazan crystals. The absorbance was measured at 570 nm using a microplate reader (Spectra Max 340; Molecular Devices, CA, USA). Finally, IC50 values were calculated. Doxorubicin was used as the positive control (Baydoun et al., 2013).
beta-Glucuronidase assay
The beta-glucuronidase assay was performed according to the method described previously, using p-nitrophenyl beta-D-glucuronide as substrate (Collins et al., 1997). The enzyme mixture (total volume 0.25 mL) contained 50 mL of p-nitrophenyl glucuronide, 190 mL of acetate buffer, 5 mL enzyme and 5 mL of inhibitor. The assay mixture was incubated at 37ºC for 40 min, the reaction was stopped by the addition of 50 mL of 0.2M Na2CO3, and the absorbance was measured at 405 nm. D-saccharic acid-1,4-lactone was used as a standard inhibitor. The percent inhibitory activity (%) was calculated using the formula; [(E-S)/EX100], where E is the activity of enzyme without test material and S is the activity of enzyme with test material.
Chemicals and reagents
Boron trifluoride solution in methanol (10%) was purchased from Fluka Chemie (Buchs, Switzerland). Sodium hydroxide solution (methanolic; 0.5N) and sodium chloride (analytical grade) were obtained from Merck (Darmstadt, Germany), while methanol (HPLC grade) and n-hexane (HPLC grade) were from Fischer Scientific (Leicestershire, UK). Helium gas (purity 99.9999%) was procured from Pak Gas (United Arab Emirates). Ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT) were from Sigma-Aldrich (Germany).
Results
Phytochemical screening
The preliminary (qualitative) phytochemical tests on T. govanianum rhizome revealed the presence of secondary metabolites in methanol extract and its fractions, such as glycosides, steroidal saponins, tannins, sterols and flavonoids (Table I). Noteworthy, samples were very rich in steroids, steroidal glycosides and saponins.
Table I: Preliminary phytochemical profile of T. govanianum
| Phytochemical | Qualitative test | Methanol extract | n-Hexane fraction | Chloroform fraction | Ethyl acetate fraction | n-Butanol fraction |
|---|---|---|---|---|---|---|
| Alkaloids | Mayer's test | - | - | - | - | - |
| Wagner's test | - | - | - | - | - | |
| Glycosides | Keller Killiani test | + | - | + | + | + |
| Tannins | Ferric chloride test | + | - | + | - | + |
| Lead acetate test | + | - | - | - | + | |
| Flavonoids | Ferric chloride test | + | - | + | - | + |
| Sodium hydroxide test | + | - | + | + | + | |
| Carbohydrates | Molisch's test | + | - | + | + | + |
| Sterols | Liebermann-Burchard test | + | + | + | + | + |
| Salkowski's test | + | + | + | + | + | |
| Saponins | Frothing test | + | - | + | + | + |
Gas chromatography/mass spectrometry (GC/MS)
The proximate fatty acid composition of n-hexane fraction was carried out by GC/MS analysis. Twelve compounds were identified by comparison of n-hexane GC/MS spectra with the mass library search (NIST based AMDIS), as shown in Table II. Unsaturated fatty acids (70%) were more abundant than saturated fatty acids (30%). Among the unsaturated fatty acids, high (C19H30O3), 9-hexadecenoic acid methyl ester (C17H32O2) and cis-13-eicosenoic acid (C20H38O2) were detected, where as 2-methyl hexadecanoic acid methyl ester (C18H36O2) and ethyl 13-methyl tetradecanoate (C17H34O2) represented the saturated fatty acids present at higher concentrations.
DPPH free radical scavenging assay
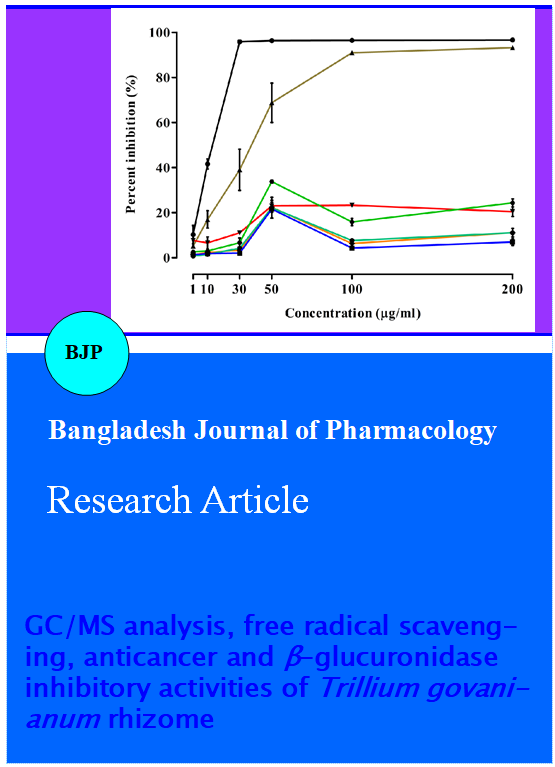
Antioxidants from natural sources are effective in reducing the toxic effects in human due to xenobiotic exposure (Wong et al., 2006). DPPH free radical scavenging assay is considered as a standard method for the assessment of the antioxidant activity of pure compounds, natural extracts and fractions (Loo et al., 2007; Lue et al., 2010; Rufino et al., 2009). Our results indicated that a higher scavenging capacity was measured in Hex-Fr and CHL-Fr (Figure 1 and 2) compared with the other fractions. This can be attributed to the presence of fatty acids, i.e. 9,12-octadecadienoic acid and hexadecanoic acid, in Hex-Fr, and glycosides, saponins and flavonoids in CHL-Fr, as previously reported on diverse plant species (Table II) (Bodoprost et al., 2007; Ha YL et al., 1990; Naga et al., 2012; Ying et al., 2014).
Figure 1: Percent of DPPH free radical scavenging activity of extract/fractions or standards (ascorbic acid and BHT). Results are mean ± SEM of three different experiments
Figure 2: DPPH free radical scavenging activity of extracts/fractions or standards (ascorbic acid and BHT). Results are mean ± SEM of three different experiments
Table II: Chemical composition of n-hexane fraction of T. govanianum
| Name | Formula | Molecular Weight |
R.T. (min) |
Abundance (%) |
|---|---|---|---|---|
| 2,4-Decadienal | C10H16O | 152 | 18.11 | 2.07 |
| Pentanoic acid-5-hydroxy-2,4-di-t-butylphenyl ester | C19H30O3 | 306 | 23.2 | 7.83 |
| Ethyl 13-methyl-tetradecanoate | C17H34O2 | 270 | 33.59 | 6.53 |
| Hexadecanoic acid methyl ester | C17H34O2 | 270 | 34.35 | 7.19 |
| 9-Hexadecenoic acid methyl ester | C17H32O2 | 268 | 35.15 | 9.65 |
| 2-Methyl hexadecanoic acid methyl ester | C18H36O2 | 284 | 35.58 | 15.00 |
| 9,12-Octadecadienoic acid methyl ester | C19H34O2 | 294 | 36.25 | 12.50 |
| 9,12-Octadecadienoic acid ethyl ester | C20H36O2 | 308 | 37.58 | 3.98 |
| 9,12-Hexadecadienoic acid methyl ester | C17H30O2 | 266 | 38.03 | 13.86 |
| 9-Octadecanoic acid methyl ester | C19H36O2 | 296 | 36.22 | 2.50 |
| cis-13-Eicosenoic acid | C20H38O2 | 310 | 39.42 | 7.27 |
| 9,12-Octadecadienoic dacid-2-hydroxy-1-(hydroxy methyl) ethyl ester | C21H38O4 | 354 | 43.25 | 4.53 |
Anticancer assay
The cytotoxic activity of methanol extract and fractions against two cancer cell lines, HeLa (cervical cancer cells) and PC-3 (prostate cancer cells), was evaluated by MTT assay. All fractions exhibited cytotoxicity against both cancer cell lines (Table III). In particular, anticancer activity of chloroform fraction on HeLa cells was slightly lower than that of doxorubicin, with IC50 of 0.8 ± 0.2 and 0.3 ± 0.0, respectively. Similarly, this fraction was also most effective against PC-3 cells (IC50 = 2.7 ± 0.3), though to a lesser extent than doxorubicin (IC50 = 1.4 ± 0.2). Moreover, the butanol fraction, although possessed moderate cytotoxic effect against the HeLa cells (IC50 = 1.6 ± 0.3) but was less effective in inhibiting the PC-3 cells (IC50 = 4.0 ± 0.3).
Table III: IC50 values of different extracts of T. govanianum and doxorubicin against cancer cells
| Extracts | IC50 (ug/mL) | |
|---|---|---|
| HeLa cells | PC-3 cells | |
| Methanol | 3.1 ± 0.7 | 6.5 ± 0.5 |
| Chloroform | 0.8 ± 0.2 | 2.7 ± 0.3 |
| Ethyl acetate | 1.4 ± 0.1 | 5.1 ± 0.3 |
| Butanol | 1.6 ± 0.3 | 4.0 ± 0.3 |
| Doxorubicin | 0.3 ± 0.0 | 1.4 ± 0.2 |
β-Glucuronidase inhibitory assay
Based on the IC50 values (µg/mL) the methanol extract (140.8 ± 3.8) and butanol fraction (196.2 ± 1.9) exhibited a moderate level of enzyme inhibitory activity in comparison to the standard D-saccharic acid-1,4-lactone (Table IV).
Discussion
In fact, plants produce a diverse range of bioactive molecules, making them a rich source of different types of medicines. A lot of research has been conducted on exploring the phytochemical and pharmacological characteristics of medicinal plants to provide a scientific rationale to their folkloric uses (Adonizio et al., 2008; Saeed et al., 2010). Keeping in view, the importance of medicinal plants and folkloric uses of T. govanianum rhizome, the present research was undertaken for the scientific exploration and validation of crude drug i.e. rhizome.
The phytochemical analysis of rhizome revealed the presence of secondary metabolites like steroids, glycolsides and saponins, and these metabolites have been previously reported in the genus Trillium (Akihito et al., 2008; Jiang et al., 2014), which includes species traditionally used in the treatment of different diseases by virtue of these phytochemicals (Abbasi et al., 2010; Andreana et al., 2002; Rahman et al., 2011; Ullah et al., 2013).
GC/MS analysis of n-hexane fraction showed the presence of saturated and unsaturated fatty acids, and thus n-hexane fraction represents the biologically active compounds with relevant antibacterial, antifungal and anticancer activities (Ching et al., 2008; Qiong et al., 2011). Therefore, the presence of these fatty acids in T. govanianum rhizome supports its potential uses as an antimicrobial and anticancer agent.
As the antioxidant activity of the extract as well as its fractions was lower than BHT and ascorbic acid. This low scavenging capacity of the extract or its fractions might be attributed to the presence of large sized fatty constituents as revealed from their phytochemical and GC-MS analyses. The DPPH assay is limited by steric accessibility, thus small molecules having low molecular weight have better access to the DPPH molecules than larger ones having high molecular weight and therefore possessed strong scavenging capacity (Bergeron et al., 2012). Moreover, there is a non-linear relationship between antioxidant activity and hydrophobicity with an increase of alkyl chain length results in low scavenging capacity (Laguerre et al., 2013).
beta-Glucuronidase enzyme has been found in animals, plants and bacteria. This enzyme catalyzes the hydrolysis of β-glucuronides conjugates of exogenous and endogenous compounds produced in the body (Fishman, 1974). From the sub-cellular fractionation studies of mammalian tissues, it was seen that beta-glucuronidase is a typical lysosomal enzyme (Fishman, 1974). Increased level of beta-glucuronides in blood has been seen in liver injury. Over expression of this enzyme may also be related to liver cancer. Similarly beta-glucuronidase of intestinal bacteria in human and rats is correlated to colon cancer. In addition to this, beta-glucuronidase of bacteria, which are found in the biliary tract is also associated with gall stone formation (Dong et al., 1999; Muhammad et al., 2002).
So far, a number of anticancer metabolites have been reported in the genus Trillium (Jiang et al., 2014; Ono et al., 2007; Ono et al., 1986) including steroidal glycosides and saponins isolated from T. erectum and T. tschonoskii (Akihito et al., 2008; Zhao et al., 2011). As the phytochemical analysis revealed the presence of steroidal glycosides, flavonoids and saponins, therefore the potent anticancer and moderate beta-glucuronidase inhibitory activity in extract and fractions of T. govanianum rhizome may be attributed due to the presence of these secondary metabolites. Therefore, the rhizomes of this plant specie may be effective in the treatment of prostate and cervical cancers and also in the management of liver and colon cancers associated with an increase activity of beta-glucuronidase.
Conclusion
To the best of our knowledge, this is the first report on the phytochemical analysis, antioxidant, beta- glucuronidase inhibitory and anti cancer activities of this plant species. These findings indicated that T. govanianum rhizome possesses a promising potential as anti-cancer medicinal plant, even if further studies, now in progress, are necessary to isolate active constituents responsible for the observed efficacy.
Acknowledgement
The authors are grateful to the H. E. J. Research Institute of Chemistry (ICCBS), University of Karachi, Pakistan, for providing necessary facilities for the bioassay.
Conflict of Interest
The authors declare no conflict of interest.
References
Abbasi AM, Khan MA, Ahmad M, Qureshi R, Arshad M, Jahan S, Zafar M, Sultana. Ethnobotanical study of wound healing herbs among the tribal communities in Northern Himalaya ranges District Abbottabad, Pakistan. Pak J Bot. 2010; 42: 3747-53.
Adonizio A, Leal SM, Ausubel FM, Mathee K. Attenuation of Pseudomonas aeruginosa virulence by medicinal plants in a Caenorhabditis elegans model system. J Med Biol. 2008; 57: 809-13.
Akihito Y, Yoshihiro M. Steroidal glycosides from the underground parts of Trillium erectum and their cytotoxic activity. Phytochemistry 2008; 69: 2724-30.
Andreana LO, Patricia L, Marian R, Michael JB, Fredi K, Adriane FB, Bonnie OC. Ethnobotanical literature survey of medicinal plants in the Dominican Republic used for women health conditions. J Ethnopharmacol. 2002; 79: 285-98.
Baydoun E, Bibi M, Iqbal MA, Atiatul W, Farran D, Smith C, Sattar SA, Attaur R, Choudhary MI. Microbial transformation of anticancer steroid exemestane and cytotoxicity of its metabolites against cancer cell lines. Chem Cent J. 2013; 7: 57.
Bergeron C, Carrier DJ, Ramaswamy S. Front matter in biorefinery coproducts: Phytochemicals, primary metabolites and value-added biomass processing. UK, John Wiley & Sons, Ltd, 2012.
Bodoprost J, Rosemeyer H. Analysis of phenacylester derivatives fatty acids from human skin surface sebum by reversed-phase HPTLC: Chromatographic mobility as a function of physicochemical properties. Int J Mol Sci. 2007; 8: 1111-24.
Botinestean C, Hadaruga NG, Hadaruga DI, Jianu I. Fatty acids composition by gas chromatography–mass spectrometry (GC-MS) and most important physical-chemicals parameters of tomato seed oil. J Agroalim Proc Tech. 2012; 18: 89-94.
Ching TH. New bioactive fatty acids. Asia Pac J Clin Nutr. 2008; 17: 192-95.
Collins RA, Ng TB, Fong WP, Wan CC, Yeung HW. Inhibition of glycohydrolase enzymes by aqueous extracts of Chinese medical herbs in a microplate format. Bioch Mol Biol Int. 1997; 42: 1163–69.
Dong K, Sang, BS, Nam JK, II-Sung J. β-Glucuronidase inhibitory activity and hepatoprotective effect of Ganoderma lucidum. Biol Pharm Bull. 1999; 22: 162-64.
Faizi S, Sumbul S, Versiani AM, Saleem R, Sana A, Siddiqui H. GC/MS-MS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac J Trop Biomed. 2014; 4: 650-54.
Fishman WH, Bergmeyer HU (ed). Methods of enzymatic analysis. 2nd edn. New York, Academic Press, 1974, pp 929–30.
Flora of Pakistan: http://www.efloras.org/florataxon.aspx?flora_id=5&taxon_id=133668, access date: 28/02/2015.
Ha YL, Storkson J, Pariza MW. Inhibition of benzo(a)pyrene induced mouse stomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990; 50: 1097–101.
Huang W, Zou K. Cytotoxicity of a plant steroidal saponin on human lung cancer cells. Asian Pac J Cancer Prev. 2011; 12: 513-17.
Jiang C, Xiaomei S, Xin W, Qibing M, Zhao Li, Jiucheng C, Zhishu T, Zhenggang Y. Two new compounds from the roots and rhizomes of Trillium tschonoskii. Phytochem Lett. 2014; 10: 113–17.
Khan SM, Page S, Ahmad H, Shaheen H, Ullah Z, Ahmad M, Harper DM. Medicinal flora and ethnoecological knowledge in Naran Valley, Western Himalaya, Pakistan. J Ethnobiol Ethnomed. 2013; 9: 4.
Laguerre M, Sorensen ADM, Bayrasy C, Lecomte J, Jacobsen C, Decker EA, Villeneuve P. Role of hydrophobicity on antioxidant activity in lipid dispersions. 2013, p 290.
Loo A, Jain K, Darah I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem. 2007; 104: 300-07.
Lue BM, Nielsen NS, Jacobsen C, Hellgren L, Guo Z, Xu X. Antioxidant properties of modified rutin esters by DPPH, reducing power iron chelation and human low density lipoprotein assays. Food Chem. 2010; 123: 221-30.
Mahmood A, Mahmood A, Malik NR, Shinwari ZK. Indigenous knowledge of medicinal plants from Gujranwala District, Pakistan. J Ethnopharmacol. 2013; 148: 714-23.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65: 55–63.
Muhammad S, Nighat A, M Aijaz A, Syed MAH, Muhammad SA, Shahida S, Atta Ur R. Chemistry and biological significance of essential oils of Cymbopogon citratus from Pakistan. Nat Prod Res. 2002; 17: 159–63.
Muhammad SK. Diversity of vascular plants, ethnobotany and their conservation status in ushairy valley, Dir Upper, NWFP, Northern Pakistan, PhD thesis, Quaid-i-Azam University, Islamabad, 2011.
Naga VKA, Venkata RB, Kasetti RB, Chippada A. Antioxidant activity and GC-MS analysis of Phragmytes vallatoria leaf ethanolic extract. Int Res J Pharm. 2012; 3: 252-54.
Okigbo RN, Anuagasi CL, Amadi JE. Advance in selected medicinal and aromatic plants indigenous to Africa. J Med Plants Res. 2009; 3: 86-95.
Ono M, Hamada T, Nohara T. A 18-norspirostanol glycosides from Trillium tschonoskii. Phytochemistry 1986; 25: 544–45.
Ono M, Sugita F, Shigematsu S, Takamura C, Yoshimitsu H, Miyashita H, Ikeda T, Nohara T. Three new steroidal glycosides from the underground parts of Trillium kamtschaticum. Chem Pharm Bull. 2007; 55: 1093–96.
Pant S, Samant SS. Ethnobotanical observations in the mornaula reserve forest of Kumoun, West Himalaya, India. Ethnobot Leaflets. 2010; 14: 193-217.
Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: A comparative overview. Evid Based Complement Alternat Med. 2005; 2: 465-74.
Qiong MX, Yan-Li L, Xiao-Ran L, Xia Li, Shi-Lin Y. Three new fatty acids from the roots of Boehmeria nivea and their antifungal activities. Nat Prod Res. 2011; 25: 640-47.
Rahman S, Ismail M, Abbasa M, Muhammad N. Study of antipyretic activity of Pistacia integerrima Stewart ex Brandis bark in Balb-C mice. J Pharm Res. 2011; 4: 4411-12.
Rufino MS, Fernandes FA, Alves R, De Brito E.S. Free radical-scavenging behaviour of some north-east Brazilian fruits in a DPPH system. Food Chem. 2009; 114: 693-95.
Saeed M, Khan H, Khan MA, Simjee SU, Muhammad N. Khan SA. Phytotoxic, insecticidal and leishmanicidal activities of aerial parts of Polygonatum verticillatum. Afr J Biotechnol. 2010; 9: 1241-44.
Savita R, Rana JC, Rana PK. Ethno medicinal plants of Chamba District, Himachal Pradesh, India. J Med Plants Res. 2013; 7: 3147-57.
Shome U, Joshi P, Sharma HP. Pharmacognostic studies on Artemisia scoparia Waldst and Kit. Proc Plant Sci. 1984; 93: 151-64.
Ullah M, Usman MK, Mahmood A, Malik NR, Hussain M, Wazir MS, Daud M, Shinwari ZK. An ethnobotanical survey of indigenous medicinal plants in Wana District South Waziristan agency, Pakistan. J Ethnopharmacol. 2013; 150: 918-24.
Wong CC, Li HB, Cheng KW, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006; 97: 705-11.
Ying C, Yonghong M, Liyong H, Juxiang Li, Haiyan S, Yuanzeng Z, Jing Y, Wenke Z. Antioxidant activities of saponins extracted from Radix trichosanthis: An in vivo and in vitro evaluation. BMC Compl Altern Med. 2014; 14; 86.
Zhao W, Gao W, Wei J, Wang Y, Huang L, Xiao P. Steroid saponins and other constituents from the rhizome of Trillium tschonoskii maxim and their cytotoxic activity. Lat Am J Pharm. 2011; 30: 1702-08.