Antioxidant, hypoglycemic and antihyperglycemic properties of Syzygium calophyllifolium
Abstract
The article discusses antioxidant oral glucose tolerance and antihyperglycemic potentials of Syzygium calophyllifolium. Extracts from successive solvent extraction were tested for the total phenolic, tannin, flavonoid content and free radical scavenging property using DPPH, ABTS+, phosphomolybdenum, FRAP, superoxide and metal chelating assays. Ethyl acetate extract of bark responded well against DPPH (IC50 4.13), ABTS+ (36832.29 µM TE/ g extract), phosphomolybdenum (100.4 g AAE/100g extract), superoxide and metal ion radicals. The methanol extract of bark was also found as an effective radical scavenger. The leaf methanol extract also showed significant antioxidant ability. The bark methanol extract could potentially reduce the blood glucose level in glucose loaded and diabetic rats. The immense antioxidant potential of S. calophyllifolium leaf and bark extracts could be taken as a good source of natural antioxidant supplement in food to defend oxidative stress related disorders like diabetes.
Introduction
Plants produce varying amount of phenolic compound. Many of these act as antioxidants by reducing the oxidation process of many biomolecules thereby inhibiting oxidative stress in human body. Polyphenols have also been associated with the health promoting effects of consuming fruits and vegetables, and this positive effect has been related with their antioxidant activity (Balasundram et al., 2006). Interest has increased in the measurement and use of plant antioxidants for scientific research as well as industrial (dietary, pharmaceutical and cosmetic) purposes. This is mainly due to their strong biological activity (Suhaj, 2006). The increasing interest in the measurement of the antioxidant activity of different plant samples has derived from the overwhelming evidence of the importance of Reactive Oxygen Species (ROS), including superoxide (OÂÂ-2), peroxyl (ROO-), alkoxyl (RO-), hydroxyl (OH-), and nitric oxide (NO-) radicals in aging and chronic diseases (Fernandes et al., 2004). In diet, even the low concentration of antioxidants significantly decreases or prevents the adverse effects of reactive species, such as reactive oxygen and nitrogen species (ROS/RNS), on normal physiological function in humans (Huang et al., 2005). Oxidative stress may complicate the diabetic condition leading to life threatening effects (Koya and King, 1998). Diabetes is usually associated with increased production of free radicals and/or impaired antioxidant defense systems, which result oxidative damage leading to ROS mediated diabetic pathogenesis (Pitozzi et al., 2003). Antioxidants of natural origin are found to reduce the risks of diabetes and associated complication (Arulmozhi et al., 2004).
Medicinal plants have always been a good source of antioxidants. Syzygium calophyllifolium of the family Myrtaceae has been reported for its profound antidiabetic effect (Gurusamy et al., 2007). Other well known species, S. cumini is widely used against diabetes (Prince et al., 1998) and oxidative stress related disorders (Banerjee et al., 2005). Meagre reports are available on the medicinal potential of this plant. The investigation will add an immense scope for developing valuable antioxidant agent as food additive.
Materials and Methods
Collection of plant materials
The leaves and bark were collected during February 2012 from Ooty district of Tamil Nadu, India. The collected plant material was identified by Dr. P. Satyanarayana and the authenticity was confirmed by comparing the voucher specimen at the herbarium of Botanical survey of India, Southern circle Coimbatore, Tamil Nadu (BSI/SRC/5/23/2012-13/Tech.453). Freshly collected plant material was cleaned to remove adhering dust and then dried under shade. The dried sample was powdered and used for further studies.
Chemicals
Ferric chloride, 2,2-diphenyl-1-picrylhydrazyl (DPPH), potassium persulfate, 2,2’aninobis(3-ethylbenzothiozoline-6-sulfonic acid) disodium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ferrous chloride, 2,4,6-tripyridyl-s-triazine (TPTZ), polyvinyl polypyrrolidone (PVPP), ethylenediamine tetracetic acid (EDTA) disodium salt streptozotocin, nicotinamide were obtained from Himedia (Mumbai, Maharashtra, India), Merck (Hyderabad, Andhra Pradesh, India) and Sigma (Thane, Maharashtra, India). All other reagents used were of analytical grade
Successive solvent extraction
The air dried, powdered plant material (100 g) was extracted in soxhlet extractor successively with petroleum ether, ethyl acetate and methanol (300 mL). Finally, the material was macerated using hot water with occasional stirring for 24 hours and the water extract was filtered. Each time before extracting with the next solvent, the material was dried in hot air oven below 40ËšC. The different solvent extracts were concentrated by rotary vacuum evaporator and then air dried. The dried extract obtained with each solvent was weighed. The percentage yield was expressed in terms of air dried weight of plant material.
Quantification of total phenolics, tannins and flavanoids
The total phenol content of leaf and bark extracts was determined according to the method described by Siddhuraju and Becker (2003). 0.5 mL of Folin-Ciocalteu reagent (1:1 with water) and 2.5 mL of sodium carbonate solution (20%) were added sequentially in each tube containing different extracts. Soon after vortexing the reaction mixture, the test tubes were placed in dark for 40 min and the absorbance was recorded at 725 nm against blank. The results are expressed as gallic acid equivalents. The extracts treated with polyvinyl polypyrrolidone (PVPP) were used for tannin estimation. 500 µL distilled water and 500 µL of the sample extracts were added to 100 mg of PVPP. The content was vortexed and kept in the test tube at 4ºC for 15 min. Then the sample was centrifuged at 4,000 rpm for 10 min at room temperature and the supernatant was collected. This supernatant has only simple phenolics other then the tannins (the tannins would have been precipitated along with the PVPP. The phenolic content of the supernatant was measured and expressed as the content of non-tannin phenolics. Siddhuraju and Manian (2007) method was followed to estimate the tannin content. The tannin content of the sample was calculated as follows:
Tannin (%) = Total phenolics (%) - Non-tannin phenolics (%)
The flavonoid contents (Zhishen et al. 1999) of all the extracts were quantified as it act as a major antioxidants in plants reducing oxidative stress. To each 500 µL of extracts 2 mL of distilled water was added. Then 150 µL of NaNO2 was added to all the test tubes followed by incubation at room temperature for 6 min. After incubition 150 µL of AlCl3 (10%) was added to all the test tubes. The test tubes were incubated for 6 min at room temperature. Then 2 mL of 4% NaOH was added to all the test tubes which were made up to 5 mL using distilled water. The contents in all the test tubes were vortexed well and they were allowed to stand for 15 min at room temperature. The pink colour developed was read spectrophotometrically at 510 nm. The amount of flavonoid was calculated in rutin equivalents.
Total antioxidant activity assay by radical cation 2, 2’-azinobis (3-ethylebenzothiozoline-6-sulphonic acid) (ABTS˙+)
ABTS radical cation decolorization assay was done to determine the total antioxidant activity of the samples according to the method of Re et al (1999) described by Siddhuraju and Manian (2007). ABTS·+ was produced by reacting 7 mM ABTS˙+ aqueous solution with 2.4 mM potassium persulphate in the dark for 12–16 hours at room temperature. The reagent solution was diluted in ethanol (about 1:89 v/v) and equilibrated at 30ºC to give an absorbance at 734 nm of 0.7 ± 0.02. After the addition of 1 mL of diluted ABTS˙+ solution to different concentration of sample or trolox standards (final concentration 0-15 µM) in ethanol, absorbance was measured at 30ºC exactly 30 min after initial mixing. Triplicate determinations were made at 734 nm and it was plotted as a function of trolox concentration. The total antioxidant activity (TAA) was expressed in µMol tolox equivalence/g extract.
Radical scavenging activity using DPPH· method
The DPPH radical scavenging activity of extracts was determined by the method of Blois (1958). A methanol solution of the sample extracts at various concentrations was added to 5 mL of 0.1 mM methonolic solution of DPPH· and allowed to stand for 20 min at 27ºC. The absorbance of the sample was measured at 517 nm. Methanol was served as blank and solution without extract served as control. The mixture of methanol, DPPH and standards (BHT, BHA, quercetin and α-rutin) served as positive control. More significantly the IC50 of the extracts were also calculated.
Ferric reducing antioxidant power (FRAP) assay:
The antioxidant capacities of phenolic extracts of samples were estimated according to the procedure described by Pulido et al. (2000). Freshly prepared FRAP reagent (2.5 mL of 20 mmol/L TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mmol/L HCl plus 2.5 mL of 20 mmol/L FeCl3·6H2O and 25 mL of 0.3 mol/L acetate buffer (pH 3.6) (900 µL) incubated at 37ºC, was mixed test sample or methanol (for the reagent blank). The test samples and reagent blank were incubated at 37ºC for 30 min in a water bath as described by Siddhuraju and Becker (2003). At the end of incubation, the absorbance readings were taken immediately at 593 nm. The FRAP value is expressed as mmol Fe (II) equivalent/mg extract.
Metal chelating activity
The chelating of ferrous ions by leaf and bark extracts was estimated by the method of Dinis et al. (1994). Briefly, 50 µL of 2 mM FeCl2 was added to the extracts. The reaction was initiated by the addition of 0.2 mL of 5 mM ferrozine solution. The mixture was vigorously shaken and left to stand at room temperature for 10 min. The absorbance of the solution was thereafter measured at 562 nm. BHT was taken as standard. All the reagents without addition of sample extract were used as negative control. The metal chelating activity is expressed in percentage of inhibition.
% inhibition = (Control OD – Sample OD / Control OD) × 100
Assay of superoxide radical (O2.- ) scavenging activity
The ability to inhibit formazan formation by scavenging superoxide radicals by the extracts was studied by the method of Beauchamp and Fridovich (1971). Each 3 mL reaction mixture (50 mM sodium phosphate buffer (pH 7.6), 20 µg riboflavin and 12 mM EDTA, and 0.1 mg NBT) with extracts in each test tubes were illuminated for 90 sec. Illuminated reaction mixture served as negative control while the mixture without extract in dark was taken as blank. Immediately after illumination, the absorbance was measured at 590 nm. The activity was compared to BHT and BHA. The percentage inhibition of superoxide anion generation was calculated using the following formula:
% inhibition = (Control OD – Sample OD / Control OD) × 100
Phosphomolybdenum assay
The antioxidant activity of samples was evaluated by the phosphomolybdenum method (Prieto et al., 1999). Sample solution was combined with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated in a water bath at 95ºC for 90 min. After cooling to room temperature, the absorbance of the mixture was measured at 765 nm against a blank. The results were reported in ascorbic acid equivalents per gram extract (AEAC).
Animals
Healthy Wistar albino rats (100-150 g) and Swiss albino mice (20-25 g) of either sex and of approximately the same age, were used for the study. They were fed with standard chow diet and water ad libitum and were housed in polypropylene cages in a well maintained and clean environment. The experimental protocol was subjected to scrutiny of institutional animal ethics committee according to CPCSEA for experimental clearance (KMCRET/PhD/04/2012-13)
Acute toxicity
Acute oral toxicity studies were performed (Ecobichon, 1997) according to the OECD (Organization for Economic Cooperation and Development) guidelines. Male Swiss albino mice (n = 6/each dose) were selected for acute toxicity study. The animals were fasted overnight with free access to water. Extract (suspended in 0.6% carboxy methyl cellulose) was administered orally at a dose of 5 mg/kg. The general behaviors such as motor activity, tremors, convulsions, straub reaction, aggressiveness, piloerection, loss of lighting reflex, sedation, muscle relaxation, hypnosis, analgesia, ptosis, lacrimation, diarrhoea and skin colour were observed for 3 days. If mortality was observed in 4/6 or 6/6 animals, then the dose administered was considered as toxic dose. However, if the mortality was observed in only one mouse out of six animals, then the dose was repeated with higher doses such as 100, 200, 500, 1000 and 2000 mg/kg.
Oral glucose tolerance test (OGTT)
For OGTT evaluation, the rats were fasted for 12 hours and blood was taken from the tail end 30 min before administration of Syzygium calophyllifolium bark (SCBM) extract (100 and 200 mg/kg). Glibenclamide was administered as standard drug (10 mg/kg). Thirty minutes later, the rats from all groups were given glucose (2 g/kg) orally. Blood were collected from the tail vein just prior to glucose administration (0 min), 30, 60, 120 and 180 min after glucose loading (Jalil et al., 2008).
Antihyperglycemic activity
Hyperglycemia was induced in overnight fasted animals by a single intraperitoneal injection of 60 mg/kg streptozotocin (STZ), 15 min after the i.p. administration of 120 mg/kg nicotinamide (Masiello et al., 1998). After 1 hour 20% glucose was orally administered to avoid the hypoglycaemic condition in diabetic rats. On 10th day, blood glucose levels were checked and the rats with glucose levels >250 mg/dL were grouped for the study. Diabetic rats were treated with oral doses of SCBM extracts (100 and 200 mg/kg) and standard glibenclamide (10 mg/kg) and blood glucose levels were observed for 5 hours.
Statistical analysis
The results were expressed as Mean ± SD/SEM. The data were statistically analyzed using SPSS version 17.0 (SPSS, ANNOVA statistical software, TULSA, USA) by means of one way ANOVA followed by Duncan’s test for antioxidant studies and Dunnett’s test for in vivo studies. Mean values were considered statistically significant when p<0.05, p<0.01 and p<0.001.
Results
The amount of total phenolics, tannins and flavonoids of different extracts of leaf, and bark of S. calophyllifolium is shown Table I. The total phenolics were found to be higher in ethyl acetate extract of bark (97.2 g GAE/100 g extract) and lowest in leaf pet ether (14.4 g GAE/100 g extract). The total tannins were found to be higher in ethyl acetate extract of bark (78.4 g GAE/ 100 g). In leaf, methanol extract (56.8 g GAE/100g extract) exhibited higher tannin content. Methanol extract of leaf was found to have appreciable flavonoid content (38.3 g RE/100g) when compared to other extracts. The least content of flavonoid was observed in leaf petroleum ether extract. The results are shown in Table I.
Table I: Total phenolic, tannin and flavonoid content of S. calophyllifolium leaf and bark
| Total phenolics (g GAE/100 g extract ) |
Tannin (g GAE/100 g extract) | Flavonoid (g RE/100 g extract ) | |
|---|---|---|---|
| Leaf | |||
| Petroleum ether | 14.4 ± 0.4 | 7.5 ± 0.5 | 2.6 ± 0.1 |
| Ethyl acetate | 41.3 ± 2.0 | 24.96 ± 2.4c | 26.0 ± 3.0b |
| Methanol | 67.8 ± 1.2c | 56.8 ± 1.3b | 38.3 ± 0.9a |
| Hot water | 50.8 ± 1.2 | 29.3 ± 1.1c | 16.8 ± 0.5 |
| Bark | |||
| Petroleum ether | 15.3 ± 0.4 | 9.7 ± 0.5 | 2.81 ± 0.0 |
| Ethyl acetate | 97.2 ± 4.5a | 78.4 ± 7.0a | 37.0 ± 0.6a |
| Methanol | 87.3 ± 6.8b | 77.3 ± 6.6a | 19.8 ± 0.5c |
| Hot water | 22.2 ± 0.5 | 13.8 ± 1.2 | 4.7 ± 0.1 |
The results of ABTS·+ cation radical scavenging activities are presented in Table II. The ethyl acetate extract of bark showed highest radical scavenging activity (36832.3 µM TE/g extract) and the least by pet ether extract of leaf (910.6 µM TE/g extract). Methanol extract of bark was also equally effective (28957.3 µM TE/g extract) in scavenging the radical. Other extracts have shown moderate activity except petroleum ether extracts.
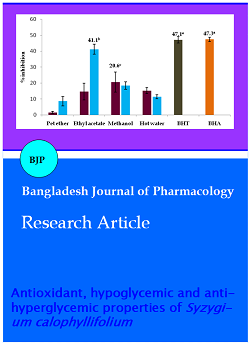
The ethyl acetate (4.1 µg/mL) and methanol (4.1 µg/mL) extract of bark showed higher DPPH· radical scavenging activity compared to other extracts. The IC50 of plant extracts are equally comparable to standards to quercetin, rutin, BHA and BHT. The DPPH radical scavenging activities are shown in Figure 1.
Figure 1: DPPH radical scavenging activity of S. calophyllifolium leaf and bark
Antioxidant potential of S. calophyllifolium was estimated from their ability to reduce TPTZ - Fe (III) complex to TPTZ- Fe (II) and is given in Table II. The ethyl acetate (9442.2 mM Fe (II)/mg extract) and methanol (9223.3 mM Fe (II)/mg extract) extracts of bark has shown higher ferric reducing activity, compared to leaf methanol extract (8982.2 mM Fe (II)/mg extract).
Table II: Activity of S. calophyllifolium leaf and bark extracts in FRAP, ABTS+ and phosphomolybdenum assays
| Solvents | FRAP (mmol Fe (II)/mg extract) |
ABTS+ (µM TE/g extract) |
Phosphomolybdenum(g AAE/100 g extract) |
|---|---|---|---|
| Leaf | |||
| Petroleum ether | 382.2 ± 25.6 | 910.6 ± 75.5 | 4.4 ± 0.0 |
| Ethyl acetate | 5793.3 ±13.3 | 18343.0 ± 911.7 | 30.0 ± 0.5 |
| Methanol | 8982.2 ± 209.5bc | 26504.9 ± 439.2b | 38.6 ± 0.5c |
| Hot water | 8832.2 ± 46.0c | 24401.1 ± 151.9c | 7.5 ± 0.2 |
| Bark | |||
| Petroleum ether | 946.7 ± 117.2 | 1192.0 ± 18.8 | 6.4 ± 0.5 |
| Ethyl acetate | 9442.2 ± 13.5a | 36832.3 ± 515.5a | 100.4 ± 0.1a |
| Methanol | 9223.3 ± 323.0ab | 28957.3 ± 957.0 | 51.3 ± 3.4b |
| Hot water | 4148.9 ± 250.1 | 9018.0 ± 455.2 | 13.9 ± 0.6 |
The Fe2+ chelating activity of extracts are shown in Figure 2. The maximum chelation was observed in the hot water (81.0%) and methanol extract of leaf (75.5%). This was much better than the standard BHT which showed a chelation of 48% (Figure 2).
Figure 2: Metal chelating activity of S. calophyllifolium leaf and bark; Values are mean of triplicate determination (n=3) ± standard deviation, where significance among the extracts p<0.05
The ability of S. calophyllifolium to inhibit formazan is directly proportional to the inhibition percentage plant extract. Ethyl acetate extract of bark found to have higher (41.1%) ability to stabilize the superoxide radicals being produced in the light, NBT and riboflavin mixture when compared to other extracts (Figure 3).
Figure 3: Superoxide radical scavenging activity of S. calophyllifolium leaf and bark; Values are mean of triplicate determination (n=3) ± standard deviation, where significance among the extracts p<0.05
The ethyl acetate extract of bark showed the highest activity (100.4 g AAE/100 g extract). Other extracts could moderately reduce the Molybdenum radicals. The results are presented in Table II.
The oral toxicity study showed the safety of the extract up to the maximum dose of 2000 mg/kg. The extract administration neither caused any significant change in the behaviours nor the death of animals in all the test groups. This indicates that SCBM was safe up to a single dose of 2000 mg/kg body weight. 1/10th and 1/20th of the maximum dose was fixed for further analyses. The animals were divided into 4 groups of six animals each for the studies. Group I standard glibenclamide, Group II 100 mg extract/kg, Group III 200 mg extract/kg and Group IV control.
The oral administrations of methanol extract of S. calophyllifolium bark enhanced the glucose tolerance in oral glucose loaded rats. The extracts (100 and 200 mg/kg) could significantly reduce the increased glucose levels in rats. The extract at 200 mg/kg could decrease the rise in glucose level by 58.9% at the end of 180 min (Figure 4).
Figure 4: Oral glucose tolerance in rats treated with SCBM; The data represent the mean ± SEM (n = 6); Significantly different at **p< 0.01; *** p<0.001, when compared to control (untreated); SCBM- S. calophyllifolium bark methanol extract
Glibenclamide showed a good ability to suppress (48.9%) the glucose load in blood. The result suggests that the diabetic rats could tolerate the blood glucose when treated with the bark methanol extract. The hypoglycaemic ability of SCBM in diabetic rats induced with STZ-nicotinamide was accessed on the 10th day. The animals showed an increase of blood glucose level above normal on 10th day. This could significantly bring down by the oral treatment of bark methanol extract at doses of 100 and 200 mg/kg. The extract showed 29.6% of reduction in blood glucose level in rats treated with 200 mg/kg at 5th hours. The standard glibenclamide could also decrease the hyperglycemic condition by 56.3% (Figure 5).
Figure 5: Antihyperglycemic activity in rats treated with SCBM;The data represent the mean ± SEM (n = 6); Significantly different at **p< 0.01; ***p<0.001, when compared to control (untreated); SCBM- S. calophyllifolium bark methanol extract
Discussion
Syzygium has a long history of being used as one of the best medicines against oxidative stress and diabetic complication both traditionally and commercially. It has been investigated in many plant species that the total phenolics could significantly contribute to the antioxidant capacity of that species. Therefore, the higher amount of phenolics in all the parts of S. calophyllifolium can be taken as a good indication for its higher antioxidant capacity. In leaf and bark, ethyl acetate and methanol extracts has shown higher total phenolic content. This clearly depicts the ability of these solvents to extract phenolics better than other organic solvents used for the study. The greater amount of tannins in the extracts of bark compared to leaf of S. calophyllifolium could be due to low free phenolics. PVPP could have precipitated the high tannin content of bark compared to that in leaf. Flavonoids are one of the most diverse and important group of natural phenolics which possess high antioxidant property. Since S. calophyllifolium possess appreciable flavonoid content, it could be assumed that the plant can have a higher free radical scavenging activity.
The ability of extracts to scavenge non biological radicals like ABTS can be best considered as the total antioxidant activity (TAA) of leaf and bark extracts that are sufficient enough to function as potential scavengers of other harmful radicals. Higher activity of bark extract has proved that the extract is rich in antioxidant compo nents that could have efficiently donated hydrogen to stabilize the radicals. DPPH radical scavenging activity was previously reported in S. cumini fruit ethanol extract by Benherlal and Arumughan (2007). This relation can definitely be attributed the activity shown by S. calophyllifolium against DPPH radical. Interestingly the extracts have shown very low IC50 values than the reference standards. The most common plant antioxidants like phenolics are also high in this plant which might have played major role converting DPPH radicals to its stable form. Iron is an essential element which is necessary for transport of oxygen molecule through blood. But under certain stress conditions these iron act as harmful free radical which will catalyze oxidative change in lipid, protein and other cellular components (Decker and Hultin 1992) which are needed to be scavenged using efficient antioxidants. From the results of DPPH assay it is quite clear that the ethyl acetate and methanol extract have compounds that could donate enough hydrogen atoms to reduce ferric ions. The higher soluble phenolics might be the reason of such ferric reducing ability. Metal chelating ability was significant as they reduce the concentration of catalyzing transition metal in lipid peroxidation (Duh et al., 1999). The activity of the methanol and hot water extract clearly exhibits the efficient chelators present in the plant. The inhibition of pink colour formation could be due to the ability of chelators to chelate the ferrous ions thereby preventing the complex formation between ferrozine and ferrous. Methanol extract of leaf also showed significant scavenging ability compared to standards BHT. Superoxide radicals can act as a precursors for the generation of most dangerous radicals like hydroxyl radicals which would results in serious damage to cellular components mainly DNA. All these investigations about this harmful radical have made it possible for us to search for the potent superoxide scavenging agent like S. calophyllifolium which can be supplemented as a natural product considering its enhanced activity. This will also reduce the risk of generating much more harmful radicals like hydrogen peroxide and hydroxyl radicals. The phosphomolybdenum reduction study was done to compare the capacity to reduce Mo (VI) to Mo (V) by the antioxidant compound present in the sample. This reduction ability was relatively shown by the active extracts of S. calophyllifolium. This reduction ability could be due to efficient electron donor present in the extracts. All these assays have clearly proved the correlation between phenolic content and the antioxidant activity active extracts.
The methanol extract of bark (SCBM) was selected for the oral glucose tolerance test and antihyperglycemic activity. This is because the extract could show a good activity equivalent to ethyl acetate and had the highest recovery percentage when extracted with methanol (30 %). Hence the use of excessive bark from the tree for extraction using ethyl acetate can be avoided. The acute toxicity study justifies that the plant can be administered for the treatment without any adverse effects. Several studies have demonstrated that hyperglycemia induced generation of free radicals and consequent development of oxidative stress mainly contributes to the development and progression of diabetes and related complications. It is predicted that hyperglycemia increases electron flux through the mitochondrial electron transport chain. Consequently, the ATP/ADP ratio increases in the mitochondria and causes the hyperpolarization of the mitochondrial membrane potential. This high electrochemical potential difference generated by the proton gradient leads to partial inhibition of the electron transport in complex III, resulting in an accumulation of electrons to coenzyme Q. This results in the incomplete reduction of O2 to generate the free radical anion superoxide (Nishikawa et al., 2000). The accelerated reduction of coenzyme Q and consequent generation of ROS is believed to be the fundamental source or mitochondrial dysfunction that plays a critical role in diabetes-related metabolic disorders and tissue histopathology (Rolo and Palmeira, 2006) Thus, reducing the risk of free radical generated oxidative stress with natural antioxidants might be an effective strategy for reducing diabetic complications (Ceriello, 2003). It could be made clear from the study that the antioxidant activity shown by the methanol extract might have influenced the reduction of blood glucose levels in oral glucose tolerance test and antihyperglycemic study. The compounds responsible for these activities need to be characterized further for proper management of oxidative stress and diabetic complication.
Conclusion
This plant could be implemented as an effective natural source of medicine for the treatment of free radical generated diseases like diabetes. Food materials supplemented with such antioxidants will also enhance the nutraceutical value in diet.
References
Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL. Neonatal streptozotocin-induced rat model of type 2 diabetes mellitus: A glance. Indian J Pharmacol. 2004; 36: 217-21.
Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006; 99: 191–203.
Banerjee A, Dasgupta N, De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 2005; 90: 727–33.
Beauchamp C, Fridovich I, Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44: 276-77.
Benherlal PS, Arumughan C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J Sci Food Agri. 2007; 87: 2560–69.
Blois MS. Antioxidants determination by the use of a stable free radical. Nature 4617 1958; 1199-200.
Ceriello A. New insights on oxidative stress and diabetic complications may lead to a causal antioxidant therapy. Diabetes Care. 2003; 26: 1589.
Decker E, Hultin HO. Lipid peroxidation in muscle foods via redox iron. In: Lipid oxidation in food. Angelo JS (ed.). Washington DC, ACS Symposium Series, 1992.
Dinis TCP, Madeira VMC, Almeida LM, Action of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophy. 1994; 315: 161–69.
Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harng Jyur (Chrysanthemum morifolium Ramat). LWT - Food Sci Technol. 1999; 32: 269-77.
Ecobichon DJ. The basis of toxicology testing. New York, CRC Press, 1997, pp 43–86.
Fernandes E, Costa D, Toste SA, Lima JLFC, Reis S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole and oxazole derivative drugs. Free Rad Biol Med. 2004; 37: 1895–905.
Gurusamy K, Kokilavani, R. Ananta Teepa KS. Effect of Syzygium calophyllifolium Walp. seed extract on transaminases and phosphatases in alloxan induced diabetic rats. Anc Sci Life. 2007; 26: 28-33.
Huang D, Ou B, Prior, RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005; 53: 1841-56.
Jalil AMM, Ismail A, Chong PP, Hamid M, Kamaruddin SHS. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (Ob-db) rats. J Agri Food Chem. 2008; 56: 7877-84.
Koya D, King GL, Protein kinase C activation and the development of diabetic complications. Diabetes. 1998; 47: 859–66.
Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire BD, Novelli M, Ribes G. Development of a new model of type 2 diabetes in adult rats administered with streptozotocin and nicotinamide. Diabetes. 1998; 47: 224–29.
Nishikawa T, Edelstein D, Brownlee M. The missing link: A single unifying mechanism for diabetic complications. Kidney Int. 2000; 58: 26–30.
Pitozzi V, Giovannelli L, Bardini G, Rotella CM, Dolara P. Oxidative DNA damage in peripheral blood cells in type 2 diabetes mellitus: Higher vulnerability of polymorphonuclear leukocytes. Mut Res. 2003; 529: 129–33.
Prieto P, Pineda M, Aguilar M. Spectophotometric quantitative of antioxidant capacity through the formation of a phosphormolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999; 269: 337–41.
Prince PSM, Menon VP, Pari L. Hypoglycemic activity of Syzygium cumini seeds: Effects on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol. 1998; 61: 1-7.
Pulido R, Bravo L, Sauro-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agri Food Chem 2000; 48: 3396-402.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice- Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. J Free Rad Biol Med. 1999; 26: 1231–37.
Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006; 212: 167–78
Siddhuraju P, Becker K. Studies on antioxidant activities of Mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino/imino acids through in vitro models. J Sci Food Agric. 2003; 83: 1517-24.
Siddhuraju R, Manian S. The antioxidant activity and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seed. Food Chem. 2007; 105: 950–58.
Suhaj M. Spice antioxidants isolation and their antiradical activity: a review. J Food Compos Anal. 2006; 19: 531–37.
Zhishen J, Mengecheng T, Jianming W. The determination of flavonoid contents on mulberry and their scavenging effects on superoxide radical. Food Chem. 1999; 64: 555-59.