Plumericin inhibits the viability of cervical cancer cells by induction of apoptosis through degradation of DNA structure
Abstract
The current study demonstrates the effect of plumericin on the viability and induction of apoptosis through degradation of double helical DNA structure in Bu25TK and HeLa cervical carcinoma cell lines. Plumericin treatment exhibited concentration- and time-dependent effect on the viability of Bu25TK and HeLa cells. Incubation of Bu25TK and HeLa cells with 40 µM concentration of plumericin decreased cell viability to 24 and 29%, respectively after 48 hours. Plumericin treatment also caused significant increase in the proportion of apoptotic cells in both the cell lines at 40 µM concentration compared to the control cells. Examination of the DNA double helical structure after 48 hours of treatment with plumericin at 40 µM concentration showed DNA degradation and formation of comets. Thus, plumericin exhibits inhibitory effect on the viability of Bu25TK and HeLa cells and induced apoptosis through DNA degradation. Therefore, plumericin is a potent candidate for the treatment of cervical carcinoma.
Introduction
Cervical cancer rate has shown increasing trend in the women of young age and comprises the second frequently detected cancer in females throughout the globe (Phongsavan et al., 2010; Wang et al., 2010). It is estimated that every year around 275,000 deaths occur due to cervical cancer and half million cases are detected (Agarwal et al., 2011). Although in most of the cervical cancer cases human papillomavirus (HPV) has been found to be associated but the cause appears not HPV alone (Kawase et al., 2010).
The mechanisms underlying the cervical cancer is yet to be fully understood (Dursun et al., 2008). The early stages of the cervical cancer are asymptomatic which results in its detection at the advanced stage (Dursun et al., 2008). At present women are advised Pap tests at regular intervals which has increased the rate of cervical cancer detection at early stage making the treatment favorable (Parkin et al., 2005; Morice and Castaigne, 2005).
The currently used strategy for the treatment involves radical surgery in combination with adjuvant chemotherapy. Despite advancement in the field of diagnostic techniques and radical surgery the rate of prognosis in the cervical carcinoma patients is very poor. Therefore, cervical cancer is considered to be the serious concern at present both in developing as well as developed countries. Thus, the discovery of novel molecules and targets for the treatment of cervical cancer is very important (Long, 2007; Brinkman et al., 2005).
Plumericin (Figure 1) has been isolated during the phytochemical investigation of the plant Plumeria bicolor in the polar medium (Little and Johnstone, 1951). The furofuranone containing natural product has been reported to exhibit wide range of biological activities including, antifungal, antibacterial, antitumor potential. The present study was aimed to investigate the effect of plumericin on the cell viability, DNA damage and apoptosis in the cervical cancer cells.
Figure 1: Chemical structure of plumericin
Materials and Methods
Cell lines
Bu25TK and HeLa cervical cell lines were purchased from American Type Culture Collection (USA). Both the cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics. Incubation of the cells was performed in an incubator at 37°C in humidified atmosphere of 5% CO2 and 95% air.
Chemicals and reagents
Plumericin was isolated during phytochemical analysis of the plant Plumeria bicolor. 4',6-diamidino-2-phenylindole (DAPI), dimethyl sulfoxide (DMSO) and N-methyl-N-nitro-N-nitrosoguanidine were purchased from Sigma-Aldrich. DMEM and propidium iodide were ordered from BD Pharmingen (USA).
Cell viability assay
Viability of Bu25TK and HeLa cervical carcinoma cells was determined by CCK-8 (Dojindo Molecular Technologies Inc.) assay. The cells were treated with different concentrations of plumericin from 10-50 µM for 12, 24, 36 and 48 hours. Briefly, the cells were distributed at a density of 2 x 106 cells per well onto the 96-well culture plates and allowed to adhere overnight. Then different concentrations of plumericin were added to each well of the 96-well plates and incubated for various time periods. After incubation, CCK-8 solution (10 µL) was put into each well. Incubation of the plates was continued for 2 hours more at 37°C followed by measurement of reading at 465 nm by using microplate reader (Bio-Rad, USA).
Analysis of apoptosis in Bu25TK and HeLa cells by flow cytometry
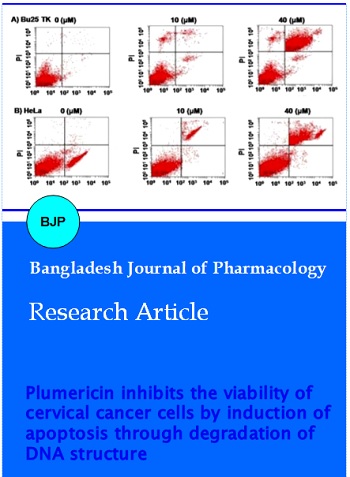
The cells were stained with an annexin V-FITC apoptosis detection kit (MBL) to determine whether cells are undergoing apoptosis. Propidium iodide staining was used as a control to differentiate cells undergoing necrosis. The cells distributed at a density of 1 x 105 onto the tissue culture slides were permitted to attach for 24 hours. The cells were then treated for 48 hours with various concentrations of plumericin and resuspended in 300 µL of 1x binding buffer. To each of the slide, annexin V-FITC (5 µL) and propidium iodide (5 µL) was added followed by incubation at 37°C for 5 min under dark conditions. Into each of slide, apoptotic cells were count using fluorescent microscopy. The effect of plumericin on the viability apoptosis in the cervical carcinoma cells. The cells after treatment with 40 µM concentration of plumericin for 48 hours were rinsed twice in PBS, fixed in 70% ethyl alcohol and subjected to propidium iodide staining. After staining, the cells were analyzed by flow cytometry (BD Facs Calibur).
DNA extraction and the detection of DNA fragmentation
Both the cervical carcinoma cell lines were incubated with 10-40 µM concentration of plumericin for 12, 24, 36 and 48 hours. Following incubation the cells were harvested, rinsed twice in phosphate-buffered saline (PBS) and subjected to DNA extraction. The DNA extraction was performed by Wizard Genomic DNA Purification kit (Promega Corporation, USA). For the purpose of analysis of fragmentation of DNA electrophoresis was carried out on denatured urea polyacrylamide gel after staining in silver nitrate.
Comet assay
The cells were seeded at a density of 2 x 106 cells onto each slide and embedded in 1% low-melting agarose. The cell lysis was then performed using lysis buffer (1.25 M NaCl, 50 mM Na-EDTA, 0.1% sodium N-lauroylsarcosine, 100 mM Tris-HCl, pH 10). The cells were digested using proteinase K (1.25 M NaCl, 5 mM Tris-HCl, 5 mM EDTA, 0.5 mg/mL proteinase K, pH 10) followed by precipitation of DNA (50% ethanol, 1 mg/mL spermine, 20 mM Tris-HCl, pH 7.4). Analysis of the breaks in single-stranded DNA was performed by soaking of slides in alkaline buffer (0.18 M NaOH, 0.6 mM NaEDTA, pH 13) followed by electrophoresis. Breaks in the double-stranded DNA were analyzed by unwinding it in neutralizing buffer (0.2% DMSO, 500 mM Na-EDTA, 100 mM Tris-HCl, 500 mM NaCl, pH 9.0) followed by electrophoresis. The cells were subjected to thidium bromide staining to assess the formation of comets. The cells were observed under light microscope connected to a digital camera having filters for 510 excitation and 590 emission. Comet Assay IV (Perspective Instruments, UK) was used for the analysis of the images.
Statistical analysis
The experiments were performed in triplicates each and results are presented as mean ± SEM. For the purpose of statistical analysis Sigma Plot 2001 software was used. p<0.05 was considered to indicate a statistically significant difference.
Results
Inhibitory effect on the cell growth
The results from CCK-8 assay demonstrated that cell viability decreased to 24 and 29% in Bu25TK and HeLa cells, respectively compared to 100% in the control with the increase in concentration of plumericin from 10 to 40 µM (Figure 2). Treatment of Bu25TK and HeLa cells with 40 µM concentration of plumericin for 12, 24 and 48 hours reduced the viability of Bu25TK cells to 86, 52 and 24%, respectively. Similarly, in HeLa cells viability was decreased to 89, 61 and 29%, respectively after 12, 24 and 48 hours (Figure 2).
Figure 2: Inhibition of cell viability in cervical carcinoma cells by plumericin. The cervical carcinoma cell lines, Bu25TK and HeLa were incubated with various concentrations of plumericin for 12, 24, 36 and 48 hours followed by analysis using MTT assay. The data presented are the mean ± S. D. of three independently performed experiments.*p<0.05, in comparison to the control cells. MTT, 3-(4,5-dimethyl- thiazol-2-yl)-2,5-diphenyltetrazolium bromide
Induces apoptosis in Bu25TK and HeLa cells
The results from flow cytometry and DNA fragmentation showed induction of apoptosis significantly in both Bu25TK and HeLa cell lines on treatment with 40 µM concentration of plumericin for 48 hours. In the cervical carcinoma cells treatment with plumericin caused formation of ladder like pattern in DNA, however, no such changes were present in the DNA of untreated cells. Fragmentation of DNA was evident in the cervical carcinoma cells after incubation with plumericin for 48 hours (Figure 3). The proportion of apoptotic cells in Bu25TK and HeLa cell cultures increased with the increase in concentration of plumericin from 10 to 40 µM (Figure 3A-D). The proportion of apoptotic cells in Bu25TK and HeLa cell cultures was found to be 73 and 69%, respectively following treatment with 40 µM concentration of plumericin for 48 hours.
Figure 3: Induction of apoptosis in Bu25TK and HeLa carcinoma cells by plumericin. (A) Analysis of apoptosis in Bu25TK and HeLa by annexin-V and PI staining. (B, C) Proportion of apoptotic cells following 48 hours of incubation with plumericin. The data presented are the mean ± S.E. of the three experiments performed independently. *p<0.05, compared with the control
Plumericin treatment induces DNA damage in Bu25TK and HeLa cells
The results from comet assay showed that plumericin treatment induced degradation of double helical DNA structure and formed comet-shaped structures. Compared to the control Bu25TK and HeLa cells, plumericin treatment caused a marked increase in the proportion of comet-positive cells (Figure 4).
Figure 4: Analysis of DNA degradation in Bu25TK and HeLa cells by comet assay. The images of the cells were captured at a magnification of x200. The cells were treated with 40 µM concentration of plumericin for 48 and N-methyl-N-nitro-N-nitrosoguanidin was taken as the control
Discussion
The present study demonstrated that plumericin treatment reduced the viability of cervical cells through induction of apoptosis by DNA damage.
Inhibition of carcinoma cell growth by treatment with natural products is considered to be the promising strategy for treatment of various cancers. The current study demonstrated that cell viability decreased significantly (p = 0.005) in Bu25TK and HeLa cells compared to the control with the increase in concentration of plumericin to 40 µM. The viability of carcinoma cells can be inhibited by induction of various cellular processes including, apoptosis, necrosis, autophagy, etc. (Bras et al., 2005; Broker et al., 2005; Edinger and Thompson, 2004). Cell apoptosis is initiated by activation of the apoptotic factors which lead to the condensation of chromatin material and degradation of the DNA double helical structure (Robertson and Orrenius, 2002). Several anti-cancer drugs exhibit their effect through apoptosis induction and DNA structure degradation. In the current study, plumericin treatment caused significant damage to the DNA in concentration dependent manner. Plumericin treatment enhanced the population of apoptotic cells significantly compared to the control cervical cells. Analysis of DNA degradation by comet assay (Cotelle and Ferard, 1999) showed increase in the percentage of comet positive cervical cells on treatment with plumericin compared to the control cells.
Conclusion
Plumericin treatment exhibits inhibitory effect on the viability of cervical carcinoma cells by causing damage to the cell DNA and inducing apoptosis. Thus, plumericin can be used as a promising candidate for the treatment cervical carcinoma.
References
Agarwal SM, Raghav D, Singh H, Raghava GP. CCDB: A curated database of genes involved in cervix cancer. Nucleic Acids Res. 2011; 39: D975-79.
Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: Different modes of dying. Biochemistry (Mosc). 2005; 70: 231-39.
Brinkman JA, Caffrey AS, Muderspach LI, Roman LD, Kast WM. Inhibitory effect of ginsenoside Rg5 and its metabolite ginsenoside Rh3 in an oxazolone induced mouse chronic dermatitis model. Eur J Gynaecol Oncol. 2005; 26: 129-42,
Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: A review. Clin Cancer Res. 2005; 11: 3155-62.
Cotelle S, Ferard JF. Comet assay in genetic ecotoxicology: A review. Environ Mol Mutagen. 1999; 34: 246-55.
Dursun P, Ayhan A, Kuscu E. New surgical approaches for the management of cervical carcinoma. Eur J Surg Oncol. 2008; 34: 487-96.
Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004; 16: 663-69.
Kawase R, Ishiwata T, Matsuda Y, et al. Expression of fibroblast growth factor receptor 2 IIIc in human uterine cervical intraepithelial neoplasia and cervical cancer. Int J Oncol. 2010; 36: 331-40.
Long HJ 3rd. Management of metastatic cervical cancer: Review of the literature. J Clin Oncol. 2007; 25: 2966-74.
Little JE, Johnstone DB. Plumericin: An antimicrobial agent from Plumeria multiflora. Arch Biochem. 1951; 30: 445-52.
Morice P, Castaigne D. Advances in the surgical management of invasive cervical cancer. Curr Opin Obstet Gynecol. 2005; 17: 5-12.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55: 74-108.
Phongsavan K, Phengsavanh A, Wahlstrom R, Marions L. Women's perception of cervical cancer and its prevention in rural Laos. Int J Gynecol Cancer. 2010; 20: 821-26.
Robertson JD, Orrenius S. Role of mitochondria in toxic cell death. Toxicology 2002; 182: 491-96.
Wang X, Liu R, Ma B, et al. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev. 2010; CD007563.