Dual receptors blocked mechanism arbitrates smooth muscles relaxant effect of Polypodium vulgare
Abstract
The study was aimed to validate the traditional uses of Polypodium vulgare in disorders associated with smooth muscle contraction and to describe its possible underlying mechanism(s) by using in vitro and in vivo experimental techniques. Rhizome's extract of P. vulgare reversed the high K+ (80 mM) and carbachol (1 µM) mediated contractions in isolated rabbit jejunum (5 and 10 mg/mL), trachea (5 and 10 mg/mL) and urinary bladder (3 and 10 mg/mL), with higher potency against carbachol than high K+, similar to dicyclomine. A rightward shift in carbachol cumulative response curves was observed in the presence of crude extract (1-3 mg/mL), similar to dicyclomine. Crude extract exhibited a dose-dependent (300-500 mg/kg) protective effect against castor oil-induced diarrhea in mice. Presence of dual blacked mechanism behind the smooth muscles relaxant effect of the crude extract, unveil medicinal significance of P. vulgare in GIT, respiratory and urinary bladder disorders.
Introduction
Polypodium vulgare Linn. (Family: Polypodiaceae) is commonly known as Bisfaij, is indigenous to Europe, Africa and Eastern Asia (Ollgaard and Tind, 1993). Plant has been used medicinally as antiepileptic (Khory and Katrak, 1981), cardiotonic, antispasmodic and digestive. It is also effective in piles, leprosy, bronchospasm, melancholia and rheumatic disorders (Dar et al., 2012). Rhizomes of P. vulgare are used as remedy for cough and common cold (Ollgaard and Tind, 1993). The plant also has nutritive values. Rhizomes of P. vulgare are used as sweetener (Dar et al., 2012).
Preliminary phytochemical studies revealed the presence of flavonoids, saponins, sterols, tannins, alkaloids, carbohydrate and phenols. P. vulgare has been reported to exhibits antioxidants (Souri et al., 2008), neuropsychopharmacological, antiepileptic, antipyretic, analgesic, hypotensive, antibiotic (Mann et al., 2008) and insecticidal (Krishnakumaran and Schneiderman, 1968) activities. Since a lot of scientific work has been done to evaluate different medicinal activities of P. vulgare, but no data is available regarding to its antispasmodic, bronchodilator and urinary bladder relaxant activities despite of its medicinal importance in these systems. The present study was conducted on the crude extract, to rationalize these folkloric uses of P. vulgare on scientific basis.
Materials and Methods
Plant material and crude extract preparation
P. vulgare rhizomes were purchased from the local herbalist in Lahore, Pakistan and specimen was submitted in the Faculty of Pharmacy, The University of Lahore, Lahore. Rhizomes were subjected to ground coarsely by an herbal grinder and then macerated in 70% hydroalcoholic solution at room temperature for 3 days with intermittently shacking as previously followed (Chaudhary et al., 2012). Macerate was filtered from a double layered muslin cloth, then from filter paper. This method was practiced again with residue to obtain maximum extract yield from soaked material. Then vaporized the filtrate in a rotary evaporator at reduced pressure (-760 mmHg) and temperature (40-45°C) to convert into a viscous mass. The semisolid extract of P. vulgare was stored in a refrigerator until used.
Drugs and chemicals
All chemicals of research grade were purchased from the authentic sources as mentioned below: Carbamylcholine chloride, dicyclomine hydrochloride and acetylcholine chloride (Sigma Chemicals Co. St Louis, MO, USA); sodium bicarbonate, sodium dihydrogen phosphate, sodium chloride, calcium chloride, potassium dihydrogen phosphate, magnesium sulphate, magnesium chloride, glucose and methanol (Merck, Darmstadt, Germany). Stock solutions of standard drugs were prepared in distilled water and stored refrigerator before use. Dilutions of standard drugs and physiological solutions (Tyrode's, Kreb's) were freshly made at the day of experiment in distilled water.
Animals
Locally breed rabbits (1.0-1.5 kg) and albino mice (25-30 g) of both sex were accommodated in the animal house of the Faculty of Pharmacy, The University of Lahore, Lahore and were provided controlled environment (23-25°C), standard diet and filtered water. Rabbits were given access to water ad libitum. They were sacrificed by blow behind the neck after 24 hours fasting.
Ex vivo experiments
Ex vivo experiments were performed on isolated rabbit jejunum, tracheal and urinary bladder preparations by following the protocol described previously (Gilani et al., 2008; Rahman et al., 2013).
Isolated rabbit jejunum
Rabbit jejunum was removed out after surgical opening of stomach and kept in a beaker filled with Tyrode's solution. Approximately 2-3 cm length segment of intestine was mounted in tissue organ bath filled with
20 mL Tyrode's solution, ventilated with carbogen and sustain at 37°C. The Tyrode's solution composition in mM was: KCl 2.68, NaCl 136.9, CaCl2 1.8, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42 and glucose 5.55 (pH 7.4). Each tissue was left untreated for 30 min to equilibrate, before the addition of any drug and then stabilized with repetitive (3-5 times) exposure of 0.3 uM acetylcholine and subsequent washing with Tyrode's solution. These experimental conditions allow the testing of the drugs effect on spontaneous rhythmic contractions of jejunum. Changes in isotonic responses of intestine were recorded through Bioscience transducer, connected to computer through a data acquisition system: Power Lab (AD Instruments, Australia).
Estimation of calcium channels blocking activity
To unveil the possible calcium channel blocking mechanism behind spasmolytic activity of crude extract, KCl (80 mM) was added to depolarize the tissue (Farre et al., 1991). Then crude extract was administered in an increasing dose pattern to observe the relaxant effect. A substance causes inhibition of contraction induced by high K+ is believed to be a Ca2+ channel blocking agent (Godfraind et al., 1986), because K+ (>30 mM) at higher concentration is known to produce contraction of smooth muscles by permitting the inflow of Ca2+ from the voltage dependent Ca2+ channels (Bolton, 1979).
Estimation of anti-cholinergic activity
To evaluate the presence of anticholinergic activity, carbachol was used to induce contraction. Carbachol mediates contraction of smooth muscle by activation of muscarinic receptors (Jenkinson, 2002). Then crude extract was added in an increasing dose pattern to observe relaxant effect (Van Rossum, 1963). A substance causes inhibition of contractions of smooth muscles mediated by carbachol, is considered to be a cholinergic blocker (Arunlakhshana and Schild, 1959).
Isolated rabbit trachea
After surgical opening trachea was removed and immersed in Kreb's solution. Isolated tracheal ring containing about two cartilages (2-3 mm wide) was cut longitudinally to form tracheal chain having smooth muscles lying in the middle of cartilage portion. Each tracheal chain was then hanged in tissue organ bath filled with 20 mL Kreb's solution, ventilated with carbogen and sustained at 37°C. The Kreb's solution composition in mM: KCl 4.7, KH2PO4 1.3, CaCl2 2.5, NaHCO3 25.0, MgSO4 1.2, NaCl 118.2 and glucose 11.7. Tissue was left untreated for 45 min under 1 g tension to equilibrated (Qureshi et al., 2015). Repetitive (2-3 time) exposure of high K+ (80 mM) or carbachol (1 uM) was given to the tissue until response was stabilized. Once the sustained contraction was achieved, crude extract was added in an increasing pattern to evaluate bronchodilator activity. Cumulative response curves were built by using increasing concentrations of carbachol. Tissue was washed after getting maximum response. Once the baseline tension was restored, the curves were rebuilt in the presence of different concentrations of the crude extract. Tissue responses were documented through isometric transducer connected to the Power Lab (AD Instruments, Australia) as described by Khan and Gilani, (2009).
Rabbit urinary bladder
Whole bladder was removed out surgically and cut vertically into 4 slices (Borchert et al., 2004). Each strip of tissue was immersed in tissue organ bath having Kreb's solution (20 mL), sustained at 37°C temperature and ventilated with carbogen. Tissue was left untreated under 1 g of tension for 45 min to equilibrate. Then high K+ or carbachol was given to the tissue until response was stabilized. Once the sustained contraction achieved, the crude extract was administered in cumulative pattern to evaluate the inhibitory effect and change in isometric responses were observed (Khan and Gilani, 2009)
In vivo experiments
Acute toxicity test
Crude extract was administered orally (10 mL/kg) in increasing doses pattern in different mice groups and allocated as test groups. Whereas negative control was given 10 mL/kg, p.o. normal saline. The mice were permitted free access to food and water, and were retained under consistent observation for 6 hours whereas mortality was noted till 24 hours (Sanmugapriya and Venkataraman, 2006).
Anti-diarrheal activity
Mice were starved 24 hours before the experiment (Borelli et al., 2006). They were separated into five groups and housed in individual cage. Group I received 10 mL/kg normal saline orally, served as negative control. Group II and III received doses of crude extract 300 and 500 mg/kg respectively, served as test groups. Group IV and V received dicyclomine (100 mg/kg) and loperamide (10 mg/kg), served as positive control. Castor oil was administered 1 hour after the treatment to induce diarrhea and observed for defecation. After that presence of characteristic diarrheal droppings were noted up to the 6 hours in individual mouse cages and anti-diarrheal effect was calculated based on the diarrheal scoring and percentage protection.
Anti-diarrheal activity was measured by calculating the percentage protection against diarrhea and the diarrheal score as follows; Score 0: No diarrhea, score 1: Soft but formed stool, score 2: Very soft stool, score 3: Very lose stool.
Preliminary phytochemical analysis
Qualitative analysis was performed for the presence of different chemical classes in the crude extract of P. vulgare (Evans and Trease, 1996; Akinyemi et al., 2005).
Statistical analysis
All experimental values were stated as mean ± SEM (standard error of mean). The EC50 values and one-way ANOVA followed by dunnett's test, was performed at confidence interval (CI) of 95%. Results were considered significant, if value p<0.05 was found.
Results
Preliminary phytochemical analysis
Qualitative analysis was performed for the presence of different chemical classes in the crude extract was found positive for the presence of alkaloids, carbohydrate, flavonoids, saponins, steroids, tannins and phenols.
Ex vivo experiments
Effects on rabbit jejunum
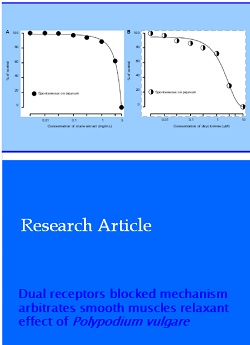
Crude extract concentration dependently (0.01-5 mg/mL) relaxed the isolated rabbit jejunum contracting spontaneously, with EC50 value of 3.2 mg/mL (2.9- 3.6; n = 4; 95% CI) (Figure 1A), similar to dicyclomine (0.01-10 µM) with EC50 value 1.9 µM (1.7 - 2.1; n = 4) (Figure 1B). When applied on high K+ and carbachol (1 µM) induced contracted rabbit jejunum, crude extract caused relaxation at 10 mg/mL and 5 mg/mL final bath concentration, with EC50 value of 5.5 mg/mL (5.1-6.0; n = 5) and 2.1 mg/mL (1.7-2.6; n = 4) respectively (Figure 1C) similar to dicyclomine with at 3 µM and 1 µM with EC50 values of 1.3 µM (1.1-1.5, n = 4) and 0.3 µM (0.2-0.4, n = 3), respectively (Figure 1D).
Figure 1: Inhibitory effect of crude extract of P. vulgare (A) and dicyclomine (B) on spontaneously contraction and the inhibitory effect of crude extract (C) and dicyclomine (D) on high K+ (80 mM) and carbachol (1 µM)-mediated contractions of isolated rabbit jejunum preparations. Values expressed with mean ± SEM; n = 3-5
Effects on rabbit trachea
When studied on high K+ or carbachol-induced contracted tracheal tissue, crude extract caused relaxation at 10 mg/mL and 5 mg/mL with EC50 values of 2.0 mg/mL (1.7-2.5; n = 4) and 2.8 mg/mL (2.5-3.1; n = 5) (Figure 2A) similar to dicyclomine at 3 µM and 1 µM with EC50 values of 1.9 µM (1.7-2.2; n = 5) and 0.4 µM (0.3-0.4; n = 3), respectively (Figure 2B). Crude extract showed concentration dependent (1-3 mg/mL) non-parallel shift towards right with the depression of maximal contractile effect in carbachol cumulative response curves, similar to dicyclomine (0.1-0.3 µM) (Figure 2C; 2D).
Figure 2: Inhibitory effect of crude extract of P. vulgare (A) and dicyclomine (B) on high K+ (80 mM) and carbachol (1 µM)-mediated contractions of isolated rabbit tracheal preparations and carbachol concentration response curve in the absence and presence crude extract (C) and dicyclomine (D) on isolated rabbit tracheal preparations. Values expressed with mean ± SEM; n = 4-5. ap<0.05; bp<0.01; cp<0.001
Effects on rabbit urinary bladder
Crude extract concentration dependently relaxed high K+ or carbachol-induced contraction in rabbit urinary bladder preparation at 10 mg/mL and 5 mg/mL with EC50 values of 1.8 mg/mL (0.9-3.4, n = 3) and 0.4 mg/mL (0.2-0.8, n = 3), respectively (Figure 3A). Similarly dicyclomine relaxed the bladder at 10 µM and 0.3 µM with EC50 values of 2.4 µM (1.2-4.5, n = 3) and 0.03 µM (0.02-0.04, n = 3) respectively (Figure 3B).
Figure 3: Inhibitory effect of crude extract of P. vulgare (A) and dicyclomine (B) on high K+ (80 mM) and carbachol (1 µM)-mediated contractions in isolated rabbit bladder preparations. Values expressed with mean ± SEM; n = 3
In vivo experiments
Acute toxicity test
Crude extract was administered in 1, 3 and 5 g/kg (body weight) doses orally to the different groups of mice. Animals were observed for any physical or behavioral changes for 24 hours but no morbidity or mortality were seen at the doses administered.
Effect on diarrhea
When studied on diarrhea induced by castor oil, loperamide showed 100% anti-diarrheal effect by decreased the diarrheal score to zero, as compare to the diseased group (score: 9.6 ± 2.79) in the mice. While crude extract showed dose dependent anti-diarrheal effect (20 and 40% protection from diarrhea) by decreasing diarrheal score 9 ± 2.3 and 7.6 ± 3.2 at dose of 300 and 500 mg/kg respectively, similar to dicyclomine 100 mg/kg that protected 60% (score: 3.6 ± 2.1) animals from diarrhea (Figure 4; Table I).
Figure 4: Bar chart showing the diarrheal score in mice groups with different treatments. Values expressed with mean ± S.E.M; n=5
Table I: Effect of crude extract of P. vulgare on diarrhea
| Treatment | No. mice/five with diarrhea | % Protection |
|---|---|---|
| Saline (10 mL/kg) + castor oil | 5/5 | 0 |
| Crude extract (300 mg/kg) + castor oil | 4/5 | 20 |
| Crude extract (500 mg/kg) + castor oil | 3/5 | 40 |
| Dicyclomine (100 mg/kg) + castor oil | 2/5a | 60 |
| Loperamide (10 mg/kg) + castor oil | 0/5b | 100 |
Discussion
Crude extract showed concentration dependently relaxation of spontaneous contraction in isolated rabbit jejunum, indicates the presence of antispasmodic effect in the crude extract. Since gastrointestinal motility is controlled by multiple physiological factors, like serotonin, histamine and acetylcholine etc and their contractile effects are linked to achieve an eventual rise in intracellular Ca2+ (Burks, 1987). To assess mechanism behind the anti-spasmodic effect, crude extract was run on high K+ or carbachol mediated contraction in isolated rabbit jejunum and a complete reversal of contractions was observed with a more pronounced effect against carbachol than high K+ similar to dicyclomine: A dual blocker of muscarinic receptor and Ca2+ channel (Downie et al., 1977). It shows the involvement of non-specific spasmolytic mechanism, most probably mediated through Ca2+ channels blockade in addition to anticholinergic constituents, present in crude extract of P. vulgare.
To endorse its folkloric claim as a bronchodilator (Dar et al., 2012), crude extract was tested on isolated rabbit tracheal preparation. Crude extract showed inhibition of carbachol mediated contraction at earlier concentration than high K+ induced contraction (Similar to pattern as shown in jejunum), suggest the presence of anticholinergic activity as predominant cause for the relaxant effect of P. vulgare. The speculation was strengthened, when crude extract showed a rightward displacement with significant suppression of maximal contractile effect in carbachol cumulative response curves (Van den, 1973) similar to dicyclomine. Since cholinergic interventions are the dominant cause of bronchoconstriction (Barnes, 1992) and the presence of anticholinergic constituents in P. vulgare provides the scientific basis for its folkloric use in respiratory disorder (Gross, 2006).
When tested on isolated rabbit urinary bladder, crude extract entirely inverted high K+ or carbachol mediated contraction and effects were comparable to those perceived in rabbit jejunum and tracheal tissues, similar to dicyclomine.
Crude extract of P. vulgare was also analyzed in vivo, because anticholinergic drugs and Ca2+ channel blocker are acknowledged to be beneficial in diarrhea (Brown and Taylor, 2006) and the speculation was strengthened when crude extract showed concentration dependent (300 and 500 mg/Kg) protection against castor oil induced diarrheal mice, comparable to dicyclomine. Since the crud extract contain both anticholinergic and Ca2+ channel blocker like constituents, so here by strongly, it can be suggested that presence of dual mode of activity (i.e. anti-cholinergic and Ca2+ channel blocker) might be responsible for anti-diarrheal effect of P. vulgare.
Preliminary phytochemical studies unveil the presence of different phytochemical classes such as alkaloids, saponins, tannins, phenoles, flavonoids and carbohydrates. Study confirms the presence of anticholinergic and Ca2+ channel blocker activity in the crude extract because alkaloids (Gilani et al., 2005; Brown and Taylor, 2006), saponins and flavonoids (Gilani et al., 1994) are known to exhibit cholinergic and Ca2+ antagonist activities, respectively.
No acute toxicity was observed when P. vulgare was administered in increasing dose pattern maximum up to 5 g/kg in the healthy mice; give the confidence about the safety of P. vulgare even used at high doses orally.
Conclusion
Smooth muscle relaxant effect of P. vulgare is mediated through a dual blockade mechanism (muscarinic receptors and Ca2+ influx) but cholinergic antagonism is more prominently, provide the pharmacological basis for the traditional uses of P. vulgare in hyperactive gastrointestinal, urinary bladder spasm and airways disorders.
Ethical Issue
Guide lines of National Research Council (NRC, 1996) were followed to perform experiments, as instructed by the animal ethics committee of the Faculty of Pharmacy, The University Lahore, Lahore, Pakistan.
References
Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005; 5: 6.
Arunlakhshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959; 14: 48-58.
Barnes PJ. Neural mechanisms in asthma. Br Med Bull. 1992; 48: 149-68.
Bolton T. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979; 59: 606-718.
Borchert VE, Czyborra P, Fetscher C, Goepel M, Michel MC. Extracts from Rhois aromatica and Solidaginis virgaurea inhibit rat and human bladder contraction. Naunyn Schmiedebergs Arch Pharmacol. 2004; 369: 281-86.
Borrelli F, Capasso F, Capasso R, Ascione V, Aviello G, Longo R, Izzo AA. Effect of Boswellia serrata on intestinal motility in rodents: Inhibition of diarrhoea without constipation. Br J Pharmacol. 2006; 148: 553-60.
Brown JH, Taylor P. Cholinergic agonists. In: The pharmacological basis of therapeutics. Brunton LL, Lazo JS, Parker KL (eds). New York, McGraw-Hill, 2006, pp 183-200.
Burks TF. Actions of drugs on gastrointestinal motility. In: physiology of the gastrointestinal tract. Johnson LR (ed). 2nd ed. New York, Raven Press, 1987.
Chaudhary MA, Imran I, Bashir S, Mehmood MH, Rehman N, Gilani AH. Evaluation of gut modulatory and bronchodilator activities of Amaranthus spinosus Linn. BMC Complement Altern Med. 2012; 12: 166.
Dar PA, Sofi G, Jafri MA. Polypodium vulgare linn. A versatile herbal medicine: A review. Int J Pharm Sci Res. 2012; 3: 1616-20.
Downie J, Twiddy D, Awad S. Anti-muscarinic and non-competitive antagonist properties of dicyclomine hydrochloride in isolated human and rabbit bladder muscle. J Pharmacol Exp Ther. 1977; 201: 662-68.
Evans WC, Trease GE. Pharmacognosy. Oxford, Alden Press, 1996.
Farre A, Colombo M, Fort M, Gutierrez B. Differential effects of various Ca2+ antagonists. Gen Pharmacol. 1991; 22: 177-81.
Gilani AH, Mehmood MH, Janbaz KH, Khan AU, Saeed SA. Ethnopharmacological studies on anti-spasmodic and anti-platelet activities of Ficuscarica. J Ethnopharmacol. 2008; 119: 1-5.
Gilani AH, Ghayur MN, Khalid A, Zaheer ul Haq, Choudhary MI, Atta ur Rahman. Presence of anti-spasmodic, anti-diarrheal, anti-secretory, calcium antagonist, acetylcholi-nesterase inhibitory, steroidal alkaloids in Sarcococca saligna. Planta med. 2005; 7: 120-25.
Gilani AH, Aftab K, Ahmed S. Cholinergic actions of crude saponins from Castanospermum australe. Pharmaboil. 1994; 32: 209-16.
Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986; 38: 321-416.
Gross NJ. Anti-cholinergic agents in asthma and COPD. Eur J Pharmacol, 2006; 533: 36-39.
Jenkinson. Classical approaches to the study of drug-receptor interactions. In: Textbook of receptor pharmacology. Foreman JC, Johansen T, (eds). Boca Raton, CRC Press, 2002, 3-78.
Khan AU, Gilani AH. Anti-spasmodic and bronchodilator activities of Artemisia vulgaris are mediated through dual blockade of muscarinic receptors and calcium influx. J Ethnopharmacol. 2009; 126: 480-86.
Khory RN, Katrak NN. Materia medica of India and their therapeutics. Delhi, Neeraj Publishing House, 1981.
Krishnakumaran A, Schneiderman H. Chemical control of moulting in arthropods. Nature 1968; 220: 601-03.
Mann A, Banso A, Clifford LC. An antifungal property of crude plant extracts from Anogeissus leiocarpus and Terminalia avicennioides. Tanzan J Health Res. 2008; 10: 34-38.
National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC, National Academy Press, 1996; 1-5.
Ollgaard B, Tind K. Scandinavian Ferns. Aarhus, Rhodos, 1993; 88-91.
Qureshi HM, Omer MO, Ashraf M, Bukhsh A, Chaudhry MA, Imran MS. Evaluation of anti-histaminic and anti-cholinergic activities of Murraya koenigii Linn. Pak Vet J. 2015; 35: 242-44.
Sanmugapriya E, Venkataraman S. Toxicological investigations on Strychnos potatorum Linn seeds in experimental animal models. J Health Sci. 2006; 52: 339-43.
Souri E, Amin G, Farsam H, Jalalizadeh H, Barezi S. Screening of thirteen medicinal plant extracts for antioxidant activity. Iran J Pharm Res. 2008; 7: 149-54.
Rahaman MS, Chaudhary MA, Ahmad B, Alamgeer A. Rationalization of traditional uses of Berberislycium in gastrointestinal disorders. Br J Med Med Res. 2013; 3: 868-79.
Van den Brink FG. Development and first check of a new model of functional synergism and antagonism. Eur J Pharmacol. 1973; 22: 270-78.
Van Rossum J. Cumulative dose-response curves II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963; 143: 299.