Occurrence of curcuminoids in Curcuma longa : A quality standardization by HPTLC
Abstract
A simple high performance thin layer chromatographic (HPTLC) method has been developed for the simultaneous determination of the pharmacologically important active curcuminoids viz. curcumin, demethoxycurcumin and bisdemethoxycurcumin in Curcuma longa L. The assay combines the separation and quantification of the analytes on silica gel 60 GF254 HPTLC plates with visualization under UV and scanning at 425 nm. Using this technique, the alkaloidal content of different parts of the title plant has been determined.
Introduction
Curcuma longa (Zingiberaceae), commonly called Haldi, is a well-known plant drug in Ayurvedic and Unani medicine (Chopra et al., 1956; Kapoor, 2001). It has been used for the treatment of various diseases and disorders particularly for urticaria, skin allergy, viral hepatitis, inflammatory conditions of joints, sore throat, and for wounds (Chattopadhyay et al., 2004).
Curcumin, demethoxycurcumin and bisdemethoxycurcumin, three major pharmacologically important curcuminoids, have been isolated from C. longa (Gupta et al., 1999) and has been shown to possess antioxidant, anti-inflammatory, anticarcinogenic, antimutagenic, antifungal, antiviral and anticancer activity (Chattopadhyay et al., 2004; Ahsan et al., 1999). Methods, so far available for the determination of these alkaloids, are very cumbersome and time-consuming and also not economically viable (Khurana and Ho, 1988; Taylor and McDowell, 1992; Schieffer, 2002).
Therefore it was thought worthwhile to develop a simple and high-precision HPTLC method for simultaneous analysis of curcumin, demethoxy curcumin and bisdemethoxycurcumin occurring in roots of C. longa.
Materials and Methods
Plant material
Rhizomes of C. longa were collected from the Experimental Farm for the Development of Medicinal and Aromatic Plants (BCKV, Mohanpur, India). The plant specimens were authenticated and a voucher specimen is deposited in the herbarium.
Chemicals and standard alkaloids
Reagents used were from Merck (Darmstadt, Germany). Analytical standards of curcuminoids were obtained from Ms. ChromaDex, Santa Ana, CA, USA. Solvents used in entire study were from Merck, India. The identities of curcumin, demethoxycurcumin and bisdemethoxycurcumin were confirmed by comparison of their spectral data with those previously reported (Govindarajan et al., 1980).
Extraction of plant material for analysis
Air dried (35-50°C) rhizomes of seven germplasm of C. longa and market turmeric powder samples (1 g each) were ultrasonically extracted separately in 20 mL HPLC grade methanol for 15 min (3 times) and filtered through Whatman No. 42 filter paper after each extraction. Extracts were concentrated under vacuum and finally made up to 20 mL with HPLC grade methanol and ready for HPTLC analysis.
Chromatographic conditions
Chromatography was performed on glass-backed silica gel 60 GF254 HPTLC layers (20 x 20 cm, 300 µm layer thickness) prepared using a Camag (Multenz, Switzerland) TLC plate auto-coater. Methanolic solutions of samples and standard compounds curcumin, demethoxycurcumin and bisdemethoxycurcumin of known concentrations were applied to the layers as 7 mm wide bands positioned 15 mm from the bottom and 20 mm from the side of the plate, using a Camag Linomat 5 automated TLC applicator with the nitrogen flow providing a delivery speed of 150 nL/s from the syringe. These parameters were kept constant throughout the analysis of samples.
Detection and quantification of the alkaloids
After sample application plates were developed in a Camag twin trough glass tank pre-saturated with the mobile phase chloroform:methanol (48:2, v/v) for one hour. It was then poured in twin trough glass solvent development chamber well in advance to allow complete saturation which was further enhanced by keeping one filter paper along one wall of the twin trough chamber. The plate was then kept in a chamber and solvent front was allowed to develop at the height of 7 cm on the plate. The TLC runs were made under laboratory conditions of 25 ± 5°C and 50% relative humidity. After drying, the spots were visualized under Camag UV cabinet (254 and 366 nm). Quantitative analysis of the compounds was done by scanning the plates using Camag TLC scanner model 3 equipped with Wincats software (Camag) applying the following conditions: slit width 6 x 0.45 mm, wavelength (λmax) 425 nm, absorption-reflection scan mode. The identifycation of curcumin, demethoxycurcumin and bisdemethoxycurcumin in rhizomes were confirmed by superimposing the UV spectra of samples and standards within the same Rf window. In order to prepare calibration curves, stock solutions of curcumin, demethoxycurcumin and bisdemethoxycurcumin (1 mg/5 mL each) were prepared and various volumes of these solutions were analyzed by HPTLC exactly as described above. Then calibration curves of peak area vs. concentration were prepared.
Results
Different compositions of the mobile phase for HPTLC analysis were tested in order to obtain high resolution and reproducible peaks. The desired aim was achieved using chloroform:methanol (48:2, v/v) as the mobile phase. The wavelength of 425 nm was found to be optimal for the highest sensitivity (Figure 1).
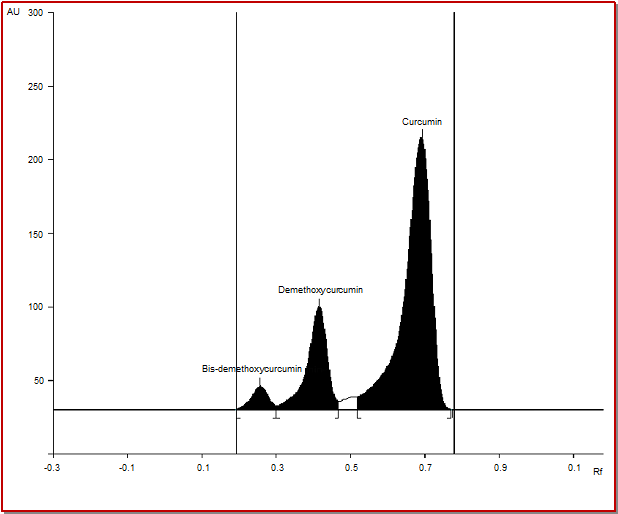
Figure 1: HPTLC chromatogram of the standard curcuminoids
The calibration curves for the alkaloids curcumin, demethoxycurcumin and bisdemethoxycurcumin were linear in the range 100-1,000 ng (Table I).
Table I: Rf values by HPTLC and linear regression equations for the determination of curcumin, demethoxycurcumin and bisdemethoxycurcumin
| Compound | Rf value | Regression equation | r |
|---|---|---|---|
| Curcumin | 0.67 | Y= 47.296+ 0.85X | 0.999 |
| Demethoxycurcumin | 0.47 | Y= 186.328+ 0.06X | 0.998 |
| Bisdemethoxycurcumin | 0.29 | Y= 271.84+ 0.39X | 0.998 |
The accuracy of the determination of the recovery rate was determined by triplicate analyses of the rhizomes spiked with three different concentrations of stock solution of curcumin, demethoxycurcumin and bisdemethoxycurcumin. The recovery rates were 97.3, 92.9 and 95.4% for curcumin, demethoxycurcumin and bisdemethoxycurcumin, respectively.
For the quantitative determination of curcumin, demethoxycurcumin and bisdemethoxycurcumin, the analyses of turmeric rhizome specimens of C. longa were repeated three times. The average content of curcumin, demethoxycurcumin and bisdemethoxycurcumin in the rhizomes are given in Table II. It is clear that three alkaloids were present in two cultivars viz. Kalimpong and PTS-43 having their maximum concentrations in the Kalimpong cultivar.
Table II: Distribution of the alkaloids curcumin, demthoxycurcumin and bisdemethoxycurcumin in two cultivars of Curcuma longa
| Cultivar | Cultivar | |
|---|---|---|
| Kalimpong | PTS-43 | |
| Curcumin (%dry weight)a | 2.19 | 1.21 |
| Demethoxycurcumin (% dry weight)a | 1.58 | 0.72 |
| Bisdemethoxycurcumin (% dry weight)a | 1.6 | 1.22 |
| aMean values (n = 3) | ||
Discussion
The HPTLC method for the simultaneous analysis of curcumin, demethoxycurcumin and bisdemethoxycurcumin from C. longa reported here is very simple, sensitive, economic and suitable for rapid screening of large number of plant samples. Moreover, this analysis can be performed without any special sample pretreatment and 15 samples can be analyzed on a single TLC laver (20 x 20 cm).
References
Ahsan H, Parveen N, Khan NU, Hadi SM. Pro-oxidant, antioxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999; 121: 161-75.
Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci. 2004; 87: 44-50.
Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi, CSTR, 1956, p 7.
Govindarajan VS. Turmeric: Chemistry, technology and quality. Crit Rev Food Sci Nutr. 1980; 12: 199-301.
Gupta AP, Gupta MM, Kumar S. Simultaneous determination of curcuminoids in curcuma samples using high performance thin layer chromatography. J Liq Chromatogr Related Technol. 1999; 22: 1561-69.
Kapoor LD. Handbook of ayurvedic medicinal plants. Boca Raton, FL, CRC Press, 2001, p 216.
Khurana A, Ho CT. High performance liquid chromatographic analysis of curcuminoids and their photo-oxidative decomposition compounds in Curcuma longa L. J Liq Chromatogr. 1988; 11: 2295-2304.
Schieffer GW. Pressurized liquid extraction of curcuminoids and curcuminoid degradation products from turmeric (Curcuma longa) with subsequent HPLC assays. J Liq Chromatogr Related Technol. 2002; 25: 3033-44.
Taylor SJ, McDowell IJ. Determination of the curcuminoid pigments in turmeric (Curcuma domestica Val.) by reversed phase high performance liquid chromatography. Chromatographia 1992; 34: 73-77.

