Preparation and evaluation of novel galantamine hydrobromide sustained-release capsule
Abstract
In present study, a novel galantamine hydrobromide sustained-release capsule was prepared with the extrusion-spheronization method and the optimized preparative formulation. The release studies were performed using marketed capsules (Razadyne ER) as a reference and data were analyzed in terms of cumulative release amounts as a function of time. Furthermore, fiber-optic real time detection was adopted to monitor the release process. Results demonstrated that our developed formulation had superior properties, worked better as sustained-release carriers and lasted longer time to release compared with the marketed product. The in vitro release characteristics of different batches of preparations were quite similar with each other, the total release proportions of galantamine hydrobromide from sustained-release capsules reached higher than 90% within 12 hours. Similar factors f2 of two preparations were all higher than 50, the release profile of drugs from capsules fitted to Higuchi model with the equation of Q% = 0.2681t1/2 + 0.0684 (r = 0.9966). Pharmacokinetics profile and parameters in beagle dogs after oral administration also revealed the superior release performances of new capsules being consistent with the in vitro study. The developed sustained-release formulation may be a promising alternative dosage form for treatment of related diseases.
Introduction
Alzheimer’s disease has become one of the most common aging diseases of the highest risk. This chronic neurodegenerative disorder is characterized by the loss of cholinergic neurons and can cause progressive impairment of memory and cognitive function, as well as the resulting behavioral disturbances (Hebert et al., 2003). Although it was suggested that the pathogenesis of Alzheimer's disease may involves oxidative injury induced by free radicals (Lipton et al., 2007; Moreira et al., 2005), cholinergic neurotransmitter system deficits (Oddo and La Ferla, 2006), and the elevated levels of proinflammatory cytokines (Wenk et al., 2002). The exact mechanism remains complicated and unknown. Now-a-days, it has been indicated that many of the memory promoters, such as brain speed shake, brain speed smoothie, and mocha focus delight etc., have chemical substances mimicking the memory promoting agents, for example, galantamine hydrobromide (Wenk, 2003).
Galantamine hydrobromide is a phenanthridine alkaloid and isolated from several members of the Amaryllidaceae family plant, such as snowdrops (de Jong et al., 2006). It can be used to moderate or delay the manifestation of Alzheimer's disease symptoms as one of the selective and reversible acetylcholinesterase (AChE) inhibitors, thus show the memory-enhancing effects (De Bruin and Pouzet, 2006). Further, its concentration-dependent inhibitive effect on AChE activity has antioxidative properties, involving decreased superoxide anion and NO overproduction, as well as restoring mitochondrial membrane potential (Ezoulin et al., 2008). Currently, among several drugs available for Alzheimer’s disease treatment, galantamine hydrobromide is the latest one recommended to improve the cognitive functions, and subsequently to treat Alzheimer's patients (U.S. National Library of Medicine, 2007). Galantamine hydrobromide has been approved most recently by the FDA for symptomatic treatment for Alzheimer's disease and vascular dementia by oral or injectable administration. However, its pharmacological activities administered by oral method or injection would be likely to cause some severe adverse effects in the gastrointestinal tract, and some organs or systems outside the central nervous system (Scharre et al., 2008; Turiiski et al., 2004). Therefore, a more efficient administration route and pharmaceutical preparation to enhance delivery ability are urgently needed (Kuna and Borra, 2013; Li et al., 2012; Marques et al., 2011).
In the present work, we intended to develop a new galantamine hydrobromide formulation, sustained-release capsule. On this basis, a simple, practical and accurate HPLC analysis method was established and validated to determine the drug contents, which was further applied to the in vitro release study. Fiber-optic real time dissolution test was applied to investigate the consistent release in batches. Furthermore, pharmacokinetics study was also conducted to verify the correlation and consistency of experimental results in vivo and in vitro. We also wished to confirm whether the release behavior of drugs could be improved by the developed samples with that of the reference.
Materials and Methods
Chemicals and reagents
The reference substances of galantamine hydrobromide (purity>99.8%, Figure 1) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Potassium dihydrogen phosphate, sodium hydroxide and triethylamine were provided by Qinjiuhong Chemical Reagent Co., Ltd (Zhengzhou, China). Hydroxypropyl methyl cellulose, microcrystalline cellulose and ethyl cellulose were provided by Beijing Huajinsheng Technology Co., Ltd. HPLC-grade methanol was purchased from Tedia Company Inc. (Fairfield, USA). The commercial product (Razadyne ER) was purchased from the market. Other chemicals of analytical grade were provided by Nanjing Chemical Reagent Co., Ltd (China). Water was distilled and purified using a Milli-Q System (Millipore, USA).
Figure 1: The chemical structure of galantamine hydrobromide
Animals
Male and female beagles (10.0 ± 0.5 kg) were supplied by Southeast University Laboratory Animal Center (Nanjing, China). Animals were kept under controlled temperature and relative humidity, and acclimatized to the housing environment for 1 week before the study. The beagles were fasted, but had free access to water overnight before the administration.
Preparation of galantamine hydrobromide sustained-release capsules
Sustained-release capsules were prepared by extrusion-spheronization method. In brief, an appropriate quantity of galantamine hydrobromide was weighed and mixed with microcrystalline cellulose, after adding a solution of hydroxypropyl methyl cellulose in water; pellets were prepared by extruding and rolling. Spray-coating technique was used to prepare immediate-release pellets with the solution of hydroxypropyl methyl cellulose on fluidized bed bottom to envelop the sealing coat, followed by taking small samples for spraying to coat with EC water dispersion on fluidized bed bottom similarly, to obtain sustained-release pellets. Finally, encapsulated the immediate-release and sustained-release ones to capsules proportionally and packed, yielded the products (Figure 2).
Figure 2: Schematic diagram of the GH sustained-release capsules manufacturing procedure. The immediate-release and sustained-release pellets were placed in a ratio of 1:3 into the capsule coating
Development of assay method
Chromatography analysis condition
The chromatography separation was performed with a diamonsil TMC18 column (200 mm x 4.6 mm, 5 µm) at a column temperature of 25°C. The mobile phase containing triethylamine phosphate buffer (PBS, pH 6.0)-methanol (75:25) was pumped at a flow rate of 1.0 mL/min, the drug was detected at 289 nm for determining the content of galantamine hydrobromide.
Specificity
The release medium, PBS (pH 6.5), blank excipients solution including hydroxypropyl methyl cellulose, microcrystalline cellulose and excipients (weighed precisely, mixed and dissolved by release medium), and the reference solution of galantamine hydrobromide were taken and injected for HPLC analysis, respectively.
Linearity
The different concentrations of galantamine hydrobromide testing solutions were prepared. The calibration curve samples were assayed in triplicate, using concentration (C) as abscissa and peak area (A) as ordinates.
Precision and accuracy
Precision and accuracy were assessed by determining the replicate QC samples (10 ug/mL) on the same day (intra-day precision) and three consecutive days (interday precision). Accuracy was described by relative error and precision was evaluated by intra- and inter-day relative standard deviation (RSD).
Recovery
Absolute recovery of analyzes was evaluated by QC samples, and data were determined by comparing the mean amounts obtained from the excipients solution spiked with reference solution with that of the neat standard samples. Three different concentration levels of analyzes were evaluated by analyzing the five samples at each level.
Stability
The stability of galantamine hydrobromide was evaluated using the samples of 100% concentration level in recovery determination experiment. The samples were analyzed at 0, 2, 4, 8, 10 and 12 hours after conditioning at room temperature, respectively.
Release rate assay method of sustained-release capsules
The oar method for dissolution test was consulted to determine release rate of galantamine hydrobromide from sustained-release capsules. 900 mL release medium was taken to dissolution glass at predetermined temperature; release medium was agitated by stirring blades at the rotation speed of 50 rpm and sampled at the scheduled time after initiating experiment. 10 mL sample was collected and filtered through a 0.45 um membrane, filtrate was selected to determine as testing solution.
Galantamine hydrobromide content at each time point was determined by HPLC analysis. Meanwhile, the proper amounts of reference substance was dissolved and diluted quantitatively by release medium to the final concentration of 10 ug/mL (8 mg standard) or 30 ug/mL (24 mg standard), which was used as standard solution for the total drug amounts (W). The above solutions were analyzed by using HPLC and external standard method, accumulative release amounts and release percent were calculated according to the formula:

Qn was the accumulative release amounts at each time point, Cn was the measured concentration at each time point, V0 was the bulk volume of release medium, Vi was the sampling volume, Ci was the measured concentration at time point i, W was the total drug amounts in capsules
Release in different medium
The in vitro release feature of galantamine hydrobromide was assessed using PBS (pH 6.5), hydrochloric acid solution (0.1M HCl), pH 4.5 buffer solution and purified water as release solvent, respectively. Release characteristics were determined at 0.5, 1, 2, 4, 6, 8, 10 and 12 hours following the assay procedures described above. Filtrate was taken for HPLC analysis to determine the accumulative release amounts at each time point and draw the release curve.
Determination for release rate
The developed formulations and reference ones (Razadyne ER, 8 mg standard in galanthamine) were both taken for release rate assessment, drug contents as well as accumulative release rate in release medium were determined. According to the guiding principles of quality standards for sustained-release, controlled release and delayed release preparations, 1, 4, and 12 hours were selected as sampling time points, the total release amounts for each capsule should attain 20-40%, 50-70% and higher than 90% corresponding to the three time points, respectively.
Drug release mechanism and statistical analysis for release data
Drug release data were analyzed by various mathematical models. The in vitro release data of galantamine hydrobromide both in developed formulations and reference products was processed by using zero-order kinetics, first-order kinetics, Higuchi, Ritger-Peppas and Weibull equations (Pradhan et al., 2014), and the data obtained from all the time points was selected to simulate. The optimum values for the parameters present in each equation were determined by linear or non-linear least-squares fitting methods. Regression analysis was performed and the best fittings were calculated on the basis of correlation coefficient as r.
According to the guideline for bioavailability and bioequiavailability of orally administered solid drugs, similarity (f2) and difference factors (f1) measuring was applied to evaluate the closeness between the two dissolution profiles (Liu et al., 2012). The f2 and f1 was calculated according to the equations given below:
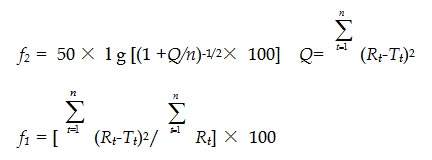
where n is the number of time points, Rt and Tt are the percentages of the reference and testing drug release at each time point t, respectively
In order to consider the release profiles similar, the f2 values should be close to 100 and the f1 values should be close to 0. In general, f2 values of the two drug release profiles is between 50 and 100, and then these two drug release characteristics are similar, whereas values below 50 indicates differences between the release profiles, similarly, f1 values within 0 and 15 show the similarity of the dissolution profiles.
Release rate determination by fiber-optic real time dissolution detection
To investigate the drug release in real-time, fiber-optic real time dissolution tester was used to determine the release rate of our developed formulations. Release assay was carried out as following: Six samples from different batches were taken into the automatism device, with PBS (pH 6.5) as release medium at the rotation speed of 50 rpm and detection wavelength was set at 289 nm. Absorbance and dissolution were assessed for 24 hours at least every 5 min and real time release rates were recorded (Li et al., 2000).
Pharmacokinetic study
The beagle dogs were randomly divided into two groups and the testing and reference preparations were orally administered to beagle dogs (24 mg), respectively. About 1 mL of blood was collected via anterior limb vein from dogs into heparinized tubes at 0.5, 1, 2, 3, 4, 6, 8, 10, 14, 18, 24, 30, 33 and 36 hours after administration (Qiu et al., 2013). Plasma was separated by centrifuging at 3,000 rpm for 10 min and stored at -70°C until analysis. The drug concentrations in plasma were determined by HPLC.
Data were processed and pharmacokinetic parameters were estimated by a non-compartmental model analysis using the Win Nonlin computer program (Version 4.0, Pharsight). Two one-sided t-test was used to compare the Cmax and Tmax of both the testing and reference preparations. Furthermore, the relative bioavailability (F) of the developed testing formulation was calculated as follows:
F =AUCt /AUCr x100%
where F is the relative bioavailability, AUCt and AUCr are the area under the concentration-time curve of developed testing formulation and marketed product (reference), respectively.
Results
Method validation
Specificity
It was indicated by the HPLC chromatograms of galantamine hydrobromide that the retention time (RT) was about 7.6 min, and no significant interferences from pharmaceutical necessities were observed in the RT of galantamine hydrobromide. Thus this method showed good specificity and selectivity for the following study under the selected conditions.
Linearity
The calibration curves were prepared at the concentration levels of 2.5-50 µg/mL and constructed with a weight of 1/x2, the typical curve equation obtained was A = 32986C-17970 with the correlation coefficient (r) 0.9999.
Precision and accuracy
The results of precision and accuracy were assessed at low, median and high levels. The RSD values of intra- and inter-day precision were within 2%, and accuracy results extended from 95 to 105%.
Recovery
Absolute recovery of galantamine hydrobromide was determined by comparing the contents of three-level QC samples incorporated with excipients to that of the standard solutions which were directly diluted by mobile phase. The recovery were 99.8 ± 0.3%, 99.6 ± 0.3% and 99.9 ± 0.3% at low, middle and high QC concentrations, respectively, showing that the absolute recovery was high enough for the analysis of in preparation.
Stability
The room temperature stability results of galantamine hydrobromide showed that the testing samples were stable under storage conditions and routine analysis for release study.
Release characteristic in different medium
The release curve of galantamine hydrobromide in four medium were shown in Figure 3. It can be drawn that the accumulative release percents calculated in different medium all reached over 90% at the last time point, suggesting that our sustained-release preparation have favorable release characteristics and the drugs can be fully released. However, it should be noted that the release of galantamine hydrobromide was affected by the medium to some extent; galantamine hydrobromide was released more rapidly within 6 hours in pH 4.5 buffer and HCl solution. The release profiles in PBS and purified water were more closed to each other and drug release in PBS was relatively more adequately in the later stages.
Figure 3: Effects of different mediums on the release of GH in sustained-release capsules. Each point represents average ± standard deviation (n=6)
Release results
The release curve of the testing and reference formulations were shown in Figure 4, contrast results of release rates were summarized in Table I. We can conclude that galantamine hydrobromide in different batches of samples was released with nearly the same property, and release rates increased continuously along with the time. Although the total release amounts were almost the same, somewhat differently, the drugs in reference samples were released more rapidly within 2 hours and reached the highest value at about 8 hours. Therefore, the results indicated that our testing capsules were of better sustained-release property comparing with the marketed product.
Figure 4: The release curves of the developed testing and marketed reference sustained-release samples, with PBS (pH 6.5) as the release medium at the rotation speed of 50 rpm
Table I: Release rate determination results of galantamine hydrobromide sustained-release capsule
| Time | 1 hour (Theoretical value of 20-40%) | 4 hours (Theoretical value of 50-70%) | 12 hours (Theoretical value >90%) |
|---|---|---|---|
| Marketed product | 34.27% ± 1.30 | 66.40% ± 0.82 | 95.10% ± 1.81 |
| Developed formulation | 31.87% ± 0.61 | 61.48% ± 0.31 | 96.33% ± 0.67 |
| Data are mean ± SD | |||
Mathematical model fitting
The release patterns of drugs from coating pellets formulations can generally be described as: The aqueous medium is absorbed by membrane that cover the drugs, thus the water permeates through the film and drugs inside the core are dissolved. With the osmotic pressure varying, water permeates into the pellets continually until the saturated condition is reached, leading to the transport processes over the membrane are transferred to diffusion in both directions. Finally, water influx and the resulting pellets swelling will induce the capsules network to expand largely, by this means, the further permeability into the film coating is promoted. As shown in Table II, through regression model parameters analysis, the drug release from testing capsules were with closest agreement with the Higuchi release kinetics (r = 0.9966), whereas Ritger-Peppas model had the best regression fitting degree for reference preparations (r = 0.9989). Furthermore, the release exponent values, “nâ€, for both the testing and marketed samples, were between 0.45 and 0.89, indicating that the drug release mechanism followed the non-Fick diffusion, which was affected by the drug diffusion and matrix corrosion.
Table II: The different release models of GH in sustained-release capsules in vitro
| Model | Equation | k | C | r | |
|---|---|---|---|---|---|
| Marketed product | Zero-order kinetics | Q% = kt + C | 0.0624 | 0.2931 | 0.969 |
| First-order kinetics | ln(100 - Q) = kt + C | -0.2336 | 4.5182 | 0.9961 | |
| Higuchi equation | Q% = kt1/2 + C | 0.27 | 0.0577 | 0.9965 | |
| Ritger-Peppas equation | lnQ = klnt + C | 0.4551 | 3.4691 | 0.9989 | |
| Weibull equation | ln [ln100/(100-Q)] = klgt + C | 1.7524 | -0.95 | 0.9903 | |
| Model | Equation | k | C | r | |
| Developed formulation | Zero-order kinetics | Q% = kt + C | 0.0622 | 0.3005 | 0.9738 |
| First-order kinetics | ln(100-Q) = kt + C | -0.2488 | 4.5488 | 0.9905 | |
| Higuchi equation | Q% = kt1/2 + C | 0.2681 | 0.0684 | 0.9966 | |
| Ritger-Peppas equation | lnQ = klnt + C | 0.4318 | 3.5165 | 0.9963 | |
| Weibull equation | ln [ln100/(100-Q)] = klgt + C | 1.7203 | -0.8984 | 0.9806 |
Data statistical analysis
The similarity factors (f2) and difference factors (f1) were calculated for evaluating the dissolution characteristic of all the formulations using the marketed product as reference. The f2 values of six batches were all higher than 50 (90.4, 92.3, 89.8, 83.9, 83.6 and 82.5, respectively), and f1 values were all lower than 15 (5.98, 1.14, 8.35, 7.85, 5.55 and 7.12, respectively), indicating that their release profiles were quite similar to that of the reference. Based on the in vitro dissolution performances, it was expected that our testing formulation and reference product may be bioequivalent, which needed further bioequivalence verification.
Release rate determination by real time dissolution detection
In situ process analysis of dissolution of galantamine hydrobromide sustained-release capsules was established by fiber-optic dissolution test system automatically. As shown in Figure 5, the dissolution curves of the six batches of samples were relatively concordant during the whole experiment; drugs were released from testing formulations steadily and the difference among batches was not significant (p>0.05). The above result showed that our developed samples had the uniform release behavior, quality stability and reliable preparation technique.
Figure 5: A comparison of the real time dissolution curve of GH sustained-release capsules of different batches. The detection was conducted automatically and real-time in six lines simultaneously, with line 1, 2, 3, 4, 5 and 6 were used for determining the samples from batch 1 to 6, respectively
Pharmacokinetic study
The plasma concentration-time distribution profiles of galantamine hydrobromide in beagle dogs were shown in Figure 6, the pharmacokinetic parameters calculated by a non-compartmental analysis using the Win Nonlin software were presented in Table III. The results showed that the pharmacokinetics of galantamine hydrobromide conformed to the two-compartment non-injective open model by program fitting. The plasma pharmacokinetic characteristics of galantamine hydrobromide in testing and reference capsules were similar, AUC0-t and Tmax were relatively closed to each other in both the preparations. However, the half-lives (T1/2) estimated in testing formulations was 6.1 ± 0.8 hours, apparently longer than that in reference ones. Similarly, mean residence time (MRT0-t) was also higher in testing capsules (7.9 ± 1.3 versus 4.6 ± 0.6), indicating that the drugs in our testing capsules can be released lasting longer in vivo. Besides, Cmax estimated in testing ones was lower than their counterparts (364.8 ± 61.4 versus 524.5 ± 41.7), which may be suggest that our pharmacy formulation for sustained-release capsules will potentially reduce the concentration fluctuate in blood levels and the following peak-valley effect. Moreover, the F value of galantamine hydrobromide from developed formulation compared with the reference calculated from AUC0-∞ was 101.1%, which indicated that the two preparations were bioequivalent. Therefore, pharmacokinetic study demonstrated that the in vitro release results can be well used to predict the in vivo bioavailability of the new formulation.
Figure 6: Concentration–time profiles in plasma of GH following oral administration of sustained-release capsules at 24 mg to beagles. Each point represents average ± standard deviation (n=5)
Table III: Pharmacokinetic parameter estimates for galantamine hydrobromide after oral administration of sustained-release capsules in beagles
| Parameter | AUC0-t (ug· h/L) | AUC0-∞ (ug· h/L) | Tmax (h) | Cmax (ug) | T1/2 (h) | MRT0-t (h) | CL (L/h/kg) | Vd (L/kg) |
|---|---|---|---|---|---|---|---|---|
| Marketed product | 2890.8 ± 1015.2 | 3042.1 ± 1046.3 | 2.4 ± 1.1 | 524.5 ± 41.7 | 4.1 ± 1.5 | 4.6 ± 0.6 | 0.01 ± 0.0 | 0.04 ± 0.0 |
| Developed formulation | 3039.1 ± 330.7 | 3075.5 ± 342.5 | 2.2 ± 0.8 | 364.8 ± 61.4 | 6.1 ± 0.8 | 7.9 ± 1.3 | 0.01 ± 0.0 | 0.1 ± 0.0 |
| Data are mean ± SD; n=5 | ||||||||
Discussion
Nowadays, the most frequently used preparative methods for sustained-release pellets formation include rolling method, centrifugalization-fluidization method, spraying-congelation method, spray drying and liquid medium method, and so on. However, extrusion-spheronization method is the most best one with the advantage of convenient operation, having the rounded, smooth and complete resulting samples of comparable size and proper rigidity (Su et al., 2012; Wang et al., 2010; Dong et al., 2013). For the post-marketing drug sample in USA, which used as control in this study, it was prepared by coating impermeable liner after fluidization dressing into the blank pellets with agent. Considering the longer dressing time is required for this method and the large drug loss resulted from spraying process, and the high cost of raw material drugs, galantamine hydrobromide, to further save the drugs, the extrusion-spheronization method was adopted by comprehensive evaluating the relative merits of all the methods (Peng et al., 2015; Lin et al., 2015).
In our previous study, preparative processes of pellets were screened to optimize with the shape, friability, content and release rate as assessment index. On the basis of these results, the trial-manufacture products were prepared in three successive batches to validate its feasibility and stability (Shibata et al., 2010; Ishida et al., 2008). The results showed that the pharmaceutical preparation technique was reasonable, convenient and controllable, thus final formulated constituents and preparation process was selected to manufacture galantamine hydrobromide immediate-release and sustained-release pellets (Ito et al., 2010; Wu et al., 2013). It should be noted that the fillings of marketed control samples consisted of immediate-release and sustained-release ones of the equal amounts for both the 8 mg and 24 mg standards, the drug loading proportion for each immediate-release to sustained-release pellet was 1:3. By contrast, we produced with immediate-release and sustained-release ones in the proportions of 1:3, which enveloped the same amounts of drugs in each small pellet, to pack and obtain our capsules within different specifications. The testing results proved that the new formulation design would result in improved sustained property.
According to the sustained-release capsule assay provided by Dissolution Methods for Drug Products and the characteristic of product, phosphate buffer (PBS, pH 6.5) was selected for release evaluation and the dissolution methodology was studied. The release rates limit values were defined based on release curve of the control samples and the guidelines for sustained and controlled-release preparation study (Pradhan et al., 2014). The experimental results indicated that 216.2 mg galantamine hydrobromide could well be dissolved in 900 mL release medium, completely matching the sink condition for release rate determination (3-7-fold of raw material drug amounts equivalent to standard amount be dissolved in the same volume of release medium) (Liu et al., 2012). The maximum specification of our test samples was 30.7 mg calculating by galantamine hydrobromide, therefore, pH 6.5 PBS can entirely meet the release determination requirement of galantamine hydrobromide in sustained-release capsules.
Generally speaking, it is somewhat difficult to characterize the dissolution rate; control the release performance and inherent quality of sustained-release preparations by a single-point dissolution test, the most frequently used in vitro release assay method (Ge et al., 2015; Lin et al., 2015). As a new-developed detection technique, the fiber-optic dissolution/release test system can monitor the real dissolution process and provide in-situ, real-time information and complete, accurate details during dissolution, through avoiding the procedure of sampling, filtering and diluting, et al, and reducing the artificial error (El-Malah et al., 2007). The automatic determining of dissolution could be accomplished simultaneously and release parameters can be directly obtained from the dissolution curves, by using real-time test system by the aid of corresponding software. Therefore, this process analysis can supply valid reference for improving preparation technology and evaluating the inherent quality of orally administered solid preparations.
Conclusion
The results indicated that the produced capsules may have better sustained-release performance, which released their contents sustaining over a longer term. On the other hand, drugs could be fully released from sustained-release carriers within the specified time limit, and there was no difference in release property between batches, being confirmed further by real-time dissolution test. Furthermore, pharmacokinetic study conducted in beagle dogs showed our developed testing capsules had better sustained-release characteristics in vivo. Comparing with the reference products, galantamine hydrobromide enveloped in testing capsules sustained above the effective plasma concentration lasting longer, were absorbed and eliminated steadier.
Ethical Issue
All studies were in compliance with the guidelines for the care and use of laboratory animals and approved by the Animal Ethics Committee of China Pharmaceutical University.
Acknowledgement
This work was financially supported by the Plan for Scientific Innovation Talent of Henan University of Technology (No: 2014CXRC07).
References
Cui Y, Zhang Y, Tang X. In vitro and in vivo evaluation of ofloxacin sustained release pellets. Int J Pharm. 2008; 360: 47–52.
De Bruin N, Pouzet B. Beneficial effects of galantamine on performance in the object recognition task in Swiss mice, deficits induced by scopolamine and by prolonging the retention interval. Pharm Biochem Behav. 2006; 85: 253–60.
de Jong CF, Derks RJE, Bruyneel B, Niessen W, Irth H. High-performance liquid chromatography-mass spectrometry based acetylcholinesterase assay for the screening of inhibitors in natural extracts. J Chromatogr A. 2006; 1112: 303–10.
Dong Q, Yi, SL, Peng, ZH, Zhao CS. The preparation and the in-vitro pharmacodynamics study of the intracapsular sustained-release preparations for the prevention of posterior capsule opaciï¬cation. Asian J Pharm Sci. 2013; 8: 252-60.
El-Malah Y, Nazzal S, Bottom CB. Hard gelatin and hypromellose (HPMC) capsules: Estimation of rupture time by real-time dissolution spectroscopy. Drug Dev Ind Pharm. 2007; 33: 27-34.
Ezoulin MJ, Ombetta JE, Dutertre-Catella H, Warnet JM, Massicot F. Antioxidative properties of galantamine on neuronal damage induced by hydrogen peroxide in SK–N–SH cells. Neuro Toxicol. 2008; 29: 270–77.
Ge SB, Peng WX, Li DL, Mo B. Minglong Zhang and Daochun Qin. Study on antibacterial molecular drugs in Eucalyptus granlla wood extractives by GC-MS. Pakistan J Pharm Sci. 2015; 28: 1445-48.
Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000°C. Arch Neurol. 2003; 60: 1119–22.
Ishida M, Abe K, Hashizume M, Kawamura M. A novel approach to sustained pseudoephedrine release: Differentially coated mini-tablets in HPMC capsules. Int J Pharm. 2008; 359: 46-52.
Ito Y, Ochii Y, Fukushima K, Sugioka N, Takada K. Three-layered microcapsules as a long-term sustained release injection preparation. Int J Pharm. 2010; 384: 53-59.
Kuna Y, Borra NK. Chronic effects of anti-Alzheimer’s drug, galantamine hydrobromide on cholinergic system of mice brain. J Pharm Res. 2013; 6: 714-19.
Lipton SA, Gu Z, Nakamura T. Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders. Int Rev Neurobiol. 2007; 82: 1–27.
Li W, Chen J, Xiang B. Simultaneous online dissolution monitoring of multicomponent solid preparations containing vitamins B1, B2, and B6 by fiberoptic sensor system. Analytica Chimica Acta. 2000; 408: 37-41.
Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmaco-kinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012; 34: 272-79.
Lin Z, Peng WX, Ge SB, Li DL, Furuta Y. Structure characteristics of oxidation pretreated fiber and biochemically binded boards against gravida abortion. J Pure Appl Microbiol. 2015; 9: 2237-42.
Lin Z, Ge SB, Li DL, Peng WX. Structure characteristics of acidic pretreated fiber and self-bind bio-boards for public health. J Pure Appl Microbiol. 2015; 9: 221-26.
Liu Y, Sun Y, Sun J, Zhao N, Sun M, He Z. Preparation and in vitro/in vivo evaluation of sustained-release venlafaxine hydrochloride pellets. Int J Pharm. 2012; 426: 21–28.
Marques LA, Maada I, de Kanter FJ, Lingeman H, Irth H, Niessen WM, Giera M. Stability-indicating study of the anti-Alzheimer’s drug galantamine hydrobromide. J Pharm Biomed Anal. 2011; 55: 85-92.
Moreira PI, Siedlak SL, Aliev G., Zhu X, Cash AD, Smith MA, Perry G. Oxidative stress mechanisms and potential therapeutics in Alzheimer disease. J Neural Transm. 2005; 112: 921–32.
Oddo S, La Ferla FM. The role of nicotinic acetylcholine receptors in Alzheimer disease. J Physiol (Paris). 2006; 99: 172–79.
Peng WX,Lin Z,Wang LS, Wu JG. Effect of Weakly Alkaline Salt Pretreatment on Bio-Boards for Medicine Safety. J Pure Appl Microbiol. 2015; 9: 1913-17.
Pradhan R, Kim YI, Chang SW, Kim JO. Preparation and evaluation of once-daily sustained-release coated tablets of tolterodine-L-tartrate. Int J Pharm. 2014; 460: 205–11.
Qiu Z, Li N, Wang X, Tian F, Liu Q, Song L, Fan Z, Lu Y, Chen X. Pharmacokinetics of vicagrel, a promising analogue of clopidogrel, in rats and beagle dogs. J Pharm Sci. 2013; 102: 741-49.
Scharre DW, Shiovitz T, Zhu Y, Amatniek J. One-week dose titration of extended release galantamine in patients with Alzheimer’s disease. Alzheimer’s Demen. 2008; 4: 30-37.
Shibata N, Nishumura A, Naruhashi K, Nakao Y, Miura R. Preparation and pharmaceutical evaluation of new sustained-release capsule including starch-sponge matrix (SSM). Biomed Pharmacother. 2010; 64: 352-58.
Su MX, Song M, Sun DZ, Zhao H, Gu X, Zhu L, Zhan XL, Xu ZN, Wen AD, Hang TJ. Determination of sinomenine sustained-release capsules in healthy Chinese volunteers by liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2012; 15: 39-43.
Turiiski VI, Krustev AD, Sirakov VN, Getova DP. In vivo and in vitro study of the influence of the anti-cholinesterase drug galantamine on motor and evacuative functions of rat gastrointestinal tract. Eur J Pharmacol. 2004; 498: 233-39.
U.S. National Library of Medicine. Donepezil, rivastigmine, galantamine. Neurology 2007; 21: 11-13.
Wang X, Fu Q, Sheng JF, Yang X, Jia JZ, Du W. Construction of a universal quantitative model for ibuprofen sustained-release capsules from different manufacturers using near-infrared diffuse reflection spectroscopy. Vib Spectrosc. 2010; 53: 214-17.
Wenk GL. Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry. 2003; 9 (64 Suppl): 7-10.
Wenk GL, Rosi S, McGann K, Hauss-Wegrzyniak B. A nitric oxide-donating flurbiprofen derivative reduces neuro-inflammation without interacting with galantamine in the rat. Eur J Pharmacol. 2002; 453: 319-24.
Wu QX, Zhang QL, Lin DQ, Yao SJ . Characterization of novel lactoferrin loaded capsules prepared with polyelectrolyte complexes. Int J Pharm. 2013; 455: 124-31.
